Chemistry Question?
2021-04-18 5:15 pm
回答 (1)
2021-04-18 5:55 pm
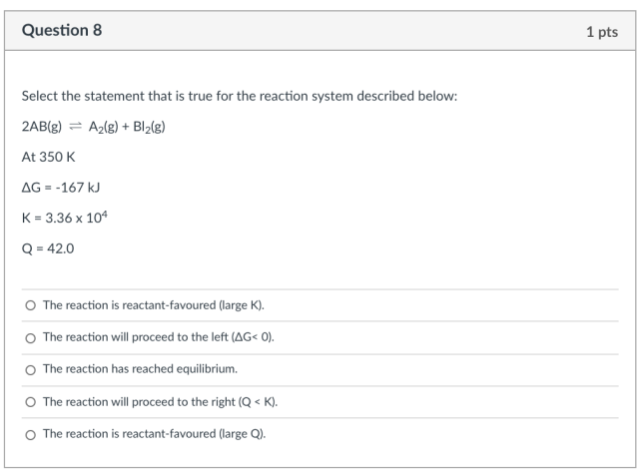
The 1st statement is false.
The reaction is "product" favoured (large K).
The 2nd statement is false.
The reaction proceed to the "right" (ΔG < 0).
The 3nd statement is false.
The reaction has not reached equilibrium because Q ≠ K.
The 4th statement is true.
The 5th statement is false.
Q does not determine which side of reaction is favoured.
The answer: The reaction will proceed to the right (Q < K). (The 4th statement)
The reaction is "product" favoured (large K).
The 2nd statement is false.
The reaction proceed to the "right" (ΔG < 0).
The 3nd statement is false.
The reaction has not reached equilibrium because Q ≠ K.
The 4th statement is true.
The 5th statement is false.
Q does not determine which side of reaction is favoured.
The answer: The reaction will proceed to the right (Q < K). (The 4th statement)
收錄日期: 2021-04-24 08:55:58
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20210418091526AAOzyXk

