What is the specific heat ( 𝑐 ) of the substance?
2021-04-02 1:06 pm
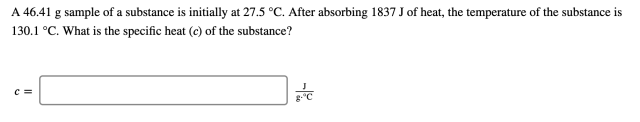
A 46.41 g sample of a substance is initially at 27.5 °C. After absorbing 1837 J of heat, the temperature of the substance is 130.1 °C. What is the specific heat ( 𝑐 ) of the substance?
回答 (1)
2021-04-02 2:06 pm
✔ 最佳答案
Heat absorbed = m c ΔT1837 J = (46.41 g) × c × [(130.1 - 27.5) °C]
c = 1837 / (46.41 × 102.6) J/g°C
Specific heat, c = 0.3858 J/g°C
收錄日期: 2021-04-24 09:51:37
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20210402050619AApU9YI

