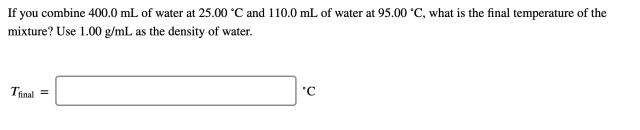
If you combine 400.0 mL of water at 25.00 ∘C and 110.0 mL of water at 95.00 ∘C, what is the final temperature of the mixture?
2021-04-02 1:04 pm
If you combine 400.0 mL of water at 25.00 ∘C and 110.0 mL of water at 95.00 ∘C, what is the final temperature of the mixture? Use 1.00 g/mL as the density of water.
回答 (1)
2021-04-02 1:58 pm
✔ 最佳答案
Let T°C be the final temperature.Heat transfer = m c ΔT
Heat absorbed by the 25.00 °C water = Heat released by the 95.00°C water
(400.0 × 1.00 g) × (c J/g°C) × [(T - 25.00) °C] = (110.0 × 1.00 g) × (c J/g°C) × [(95.00 - T) °C]
40 × (T - 25) = 11 × (95 - T)
40T - 1000 = 1045 - 11T
51T = 2045
T = 40.10
Final temperature = 40.10 °C
收錄日期: 2021-04-25 13:43:10
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20210402050445AAAXZJG

