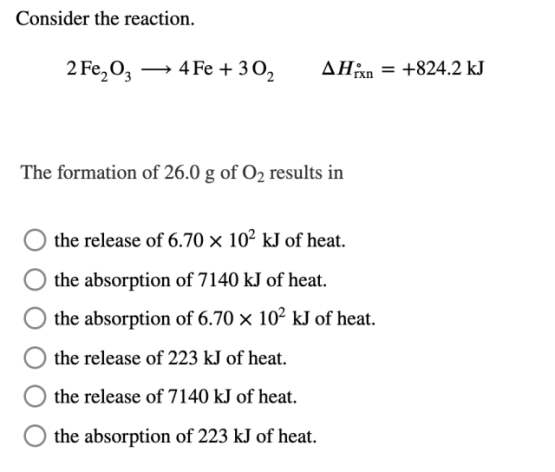
Consider the reaction. 2Fe2O3⟶4Fe+3O2Δ𝐻∘rxn=+824.2 kJ The formation of 26.0 g of O2 results in?
2021-03-24 10:41 am
回答 (1)
2021-03-24 10:56 am
✔ 最佳答案
Molar mass of O₂ = 16.0×2 g/mol = 32.0 g/molMoles of O₂ formed = (26.0 g) / (32.0 g/mol) = 0.8125 mol
2 Fe₂O₃ → 4 Fe + 3 O₂ ΔHrxn° = 824.2 kJ
On formation of 3 moles of O₂, 824.2 kJ of heat is absorbed.
(824.2 kJ heat absorbed / 3 mol O₂) × (0.8125 mol O₂)
= 223 kJ heat absorbed
The answer: The absorption of 223 kJ of heat (the last option)
收錄日期: 2021-04-24 09:50:55
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20210324024129AAQwbWJ

