更新1:
I appreciate if anyone can help me in this case also https://answers.yahoo.com/question/index?qid=20210321195840AAuldi1
I appreciate if anyone can help me in this case also https://answers.yahoo.com/question/index?qid=20210321195840AAuldi1
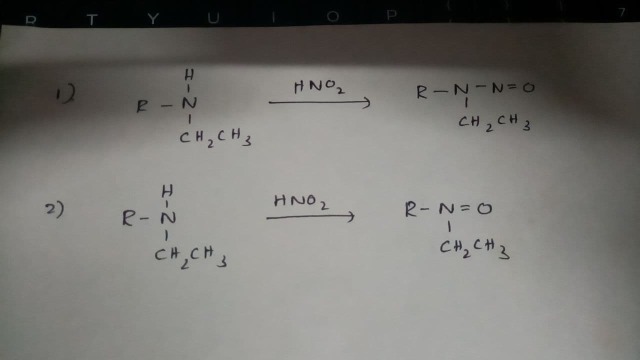
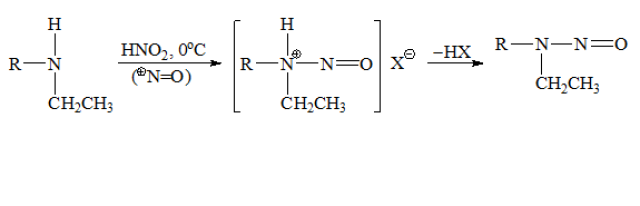
收錄日期: 2021-04-23 23:07:24
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20210322134821AAXKRSM