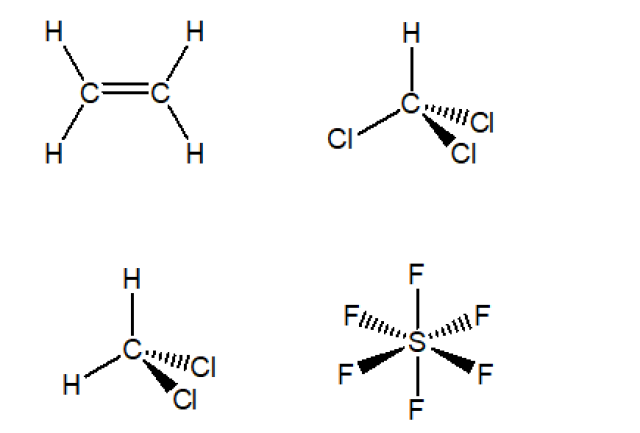
H2C = CH2
CH Cl3
CH2 Cl2
SF6
Which of the following compounds is likely to have a dipole moment?
2021-02-09 9:01 am
回答 (1)
2021-02-09 10:38 am
✔ 最佳答案
The 3-dimensional structures of the four compounds are shown below.Each of H₂C=CH₂ and SF₆ does not have a dipole moment because the dipole moments of the bonds cancel each other.
Each of CHCl₃ and CH₂Cl₂ has a dipole moment because the dipole moments of the bonds cannot cancel each other.
The answers: CHCl₃ and CH₂Cl₂
收錄日期: 2021-04-23 23:07:22
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20210209010118AALzV0D

