Just trying to work ahead on my o-chem class. Am I doing this correctly?
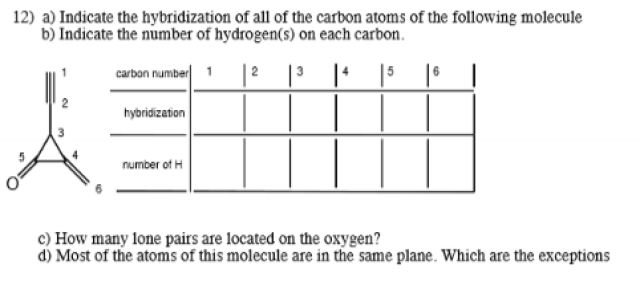
a)1 - linear2 - linear3 - tetrahedral4 - trigonal planar5 - trigonal planar6 - trigonal planarb) 1,0,1,0,0,2c) 2d) not sure!
Organic Chemistry Study Help?
2021-01-04 11:41 am
回答 (1)
2021-01-04 3:48 pm
✔ 最佳答案
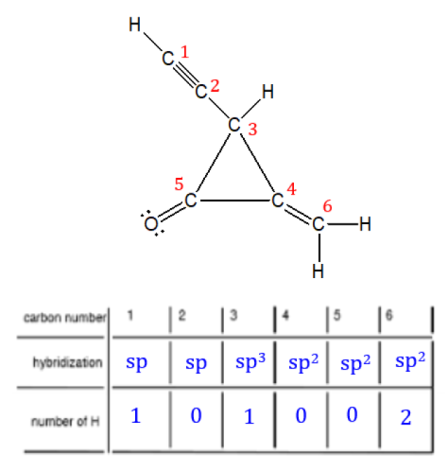
The detailed structural formula of the compound is shown below.a)
Refer to the table below.
(sp hybridization - linear)
(sp² hybridization - trigonal planar)
(sp³ hybridization - tetrahedral)
b)
Refer to the table below.
c)
Number of lone pairs local on the oxygen = 2
4)
Exceptions: The -H atom attached to C₃ and all the atoms in -C≡CH group.
(The atoms involving sp2 hybridization lie on the same plane.)
(The C atoms in a 3-membered ring lie on the same plane.)
收錄日期: 2021-04-23 23:08:30
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20210104034145AAcghXq

