What is the VSEPR for IF3?
2020-11-04 12:41 am
回答 (2)
2020-11-04 1:13 am
✔ 最佳答案
In IF₃, the central atom I has 3 I−F bonds and 2 lone pairs of electrons. According to VSEPR, the 5 electron pairs are arranged as trigonal bipyramidal in shape, and the molecule is trigonal planar in shape. The shape of IF₃ is shown in the figure below. 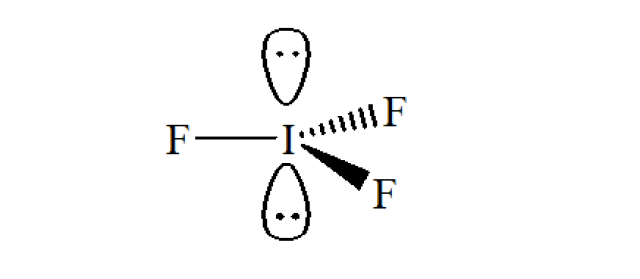
2020-11-04 1:00 am
Sorry - I don't feeling like doing your homework also today.
收錄日期: 2021-04-25 13:39:57
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20201103164110AAIiBgK

