This stoichiometry question in confusing somebody explain to me please. Question in below.?
2020-09-29 9:33 pm
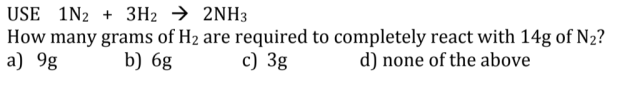
回答 (1)
2020-09-29 10:48 pm
✔ 最佳答案
1 N₂ + 3 H₂ → 2 NH₃Mole ratio N₂ : H₂ = 1 : 3
No. of moles of N₂ reacted = (14 g) / (28 g/mol) = 0.5 mol
No. of moles of H₂ reacted = (0.5 mol) × 3 = 1.5 mol
Mass of H₂ = (1.5 mol) × (2 g/mol) = 3 g
The answer: c) 3 g
====
OR:
(14 g N₂) × (1 mol N₂ / 28 g N₂) × (3 mol H₂ / 1 mol N₂) × (2 g H₂ / 1 mol H₂)
= 3 g N₂
The answer: c) 3 g
收錄日期: 2021-04-24 08:01:16
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200929133314AAu42SG
