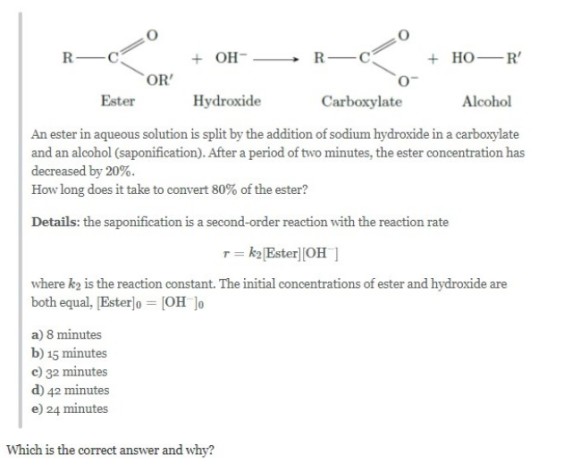
Question pertaining to alkaline hydrolysis of ester.?
2020-09-29 3:06 pm
回答 (1)
2020-09-29 6:03 pm
✔ 最佳答案
The reaction is second-order: r = k₂[Ester][OH⁻]As [Ester]ₒ = [OH⁻] and mole ratio Ester: OH⁻ = 1 : 1, [Ester] = [OH⁻] at all time t.
Hence, the rate equation can be written as: r = k₂[Ester]²
The integrated form of the rate law: 1/[Ester] = kt + (1/[Ester]ₒ)
When t = 2 min, [Ester] = (1 - 20%)[Ester]ₒ = 0.8[Ester]ₒ:
1/(0.8[Ester]ₒ) = k(2) + (1/[Ester]ₒ)
1.25/[Ester]ₒ = 2k + (1/[Ester]ₒ)
2k = 0.25/[Ester]ₒ
k = 0.125/[Ester]ₒ …… [1]
When [Ester] = (1 - 80%)[Ester]ₒ = 0.2[Ester]:
1/(0.2[Ester]ₒ) = kt + (1/[Ester]ₒ)
5/[Ester]ₒ = kt + (1/[Ester]ₒ)
kt = 4/[Ester]ₒ …… [2]
[2]/[1]:
t = 4/0.125 min
Time taken to convert 80% of the ester, t = 32 min
The answer: c. 32 minutes
收錄日期: 2021-04-24 08:00:56
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200929070644AAXR5AI
