The density of palladium is 12.0 g/cm 3. The unit cell of Pd is a face-centered cube. Calculate the atomic radius of Pd?
2020-09-21 3:04 pm
回答 (2)
2020-09-21 4:38 pm
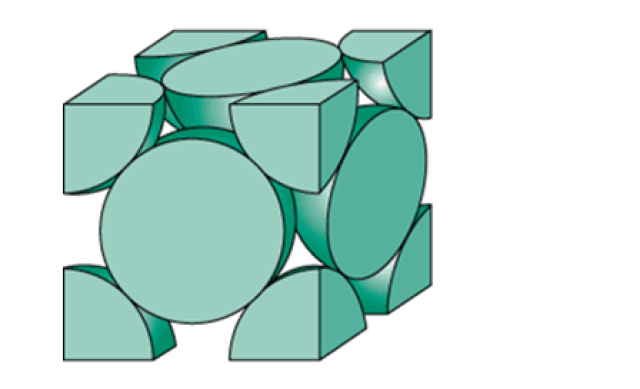
The figure below shows a unit cell of face-centered cube of Pd.
No. of Pd atoms at the corners = 8 × (1/8) = 1
No. of Pd atoms on the faces = 6 × (1/2) = 3
No. of Pd atoms in an unit cell = 1 +m 3 = 4
The mass of each mole (6.022 × 10²³ atoms) of Pb = 106.4 g
Mass of an unit cell of Pd = (106.4 g) × [4/(6.022 × 10²³)] = 7.067 × 10⁻²² g
Volume of an unit cell of Pd = (7.067 × 10⁻²² g) / (12.0 g/cm³) = 5.889 × 10⁻²³ cm³
Length of each edge of an unit cell of Pd = √(5.889 × 10⁻²³ cm³) = 3.891 × 10⁻⁸ cm
Length of the diagonal of each face of the unit cell of Pd:
4 × (Radius of Pd atom) = (3.891 × 10⁻⁸ cm) × √2
Radius of Pd atom = (1/4) × (3.891 × 10⁻⁸) × √2 cm = 1.38 × 10⁻⁸ cm = 13.8 × nm
No. of Pd atoms at the corners = 8 × (1/8) = 1
No. of Pd atoms on the faces = 6 × (1/2) = 3
No. of Pd atoms in an unit cell = 1 +m 3 = 4
The mass of each mole (6.022 × 10²³ atoms) of Pb = 106.4 g
Mass of an unit cell of Pd = (106.4 g) × [4/(6.022 × 10²³)] = 7.067 × 10⁻²² g
Volume of an unit cell of Pd = (7.067 × 10⁻²² g) / (12.0 g/cm³) = 5.889 × 10⁻²³ cm³
Length of each edge of an unit cell of Pd = √(5.889 × 10⁻²³ cm³) = 3.891 × 10⁻⁸ cm
Length of the diagonal of each face of the unit cell of Pd:
4 × (Radius of Pd atom) = (3.891 × 10⁻⁸ cm) × √2
Radius of Pd atom = (1/4) × (3.891 × 10⁻⁸) × √2 cm = 1.38 × 10⁻⁸ cm = 13.8 × nm
2020-09-21 7:46 pm
The last statement of the given solution is this:
Radius of Pd atom = (1/4) × (3.891 × 10⁻⁸) × √2 cm = 1.38 × 10⁻⁸ cm = 13.8 × nm
The typical unit for atomic radii is picometers, not nanometers.
1.38 × 10⁻⁸ cm = 138 pm
By the way, the answer in nm is 0.138 nm. I'm not sure what that ending part of 13.8 × nm is doing there.
Everything else on the calculation is just fine. For some more examples, please go here:
https://www.chemteam.info/Liquids&Solids/WS-fcc-AP.html
Radius of Pd atom = (1/4) × (3.891 × 10⁻⁸) × √2 cm = 1.38 × 10⁻⁸ cm = 13.8 × nm
The typical unit for atomic radii is picometers, not nanometers.
1.38 × 10⁻⁸ cm = 138 pm
By the way, the answer in nm is 0.138 nm. I'm not sure what that ending part of 13.8 × nm is doing there.
Everything else on the calculation is just fine. For some more examples, please go here:
https://www.chemteam.info/Liquids&Solids/WS-fcc-AP.html
收錄日期: 2021-04-24 08:00:21
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200921070438AASQh40
