Help with chemistry questions ?
2020-09-10 12:57 am
回答 (2)
2020-09-10 1:54 am
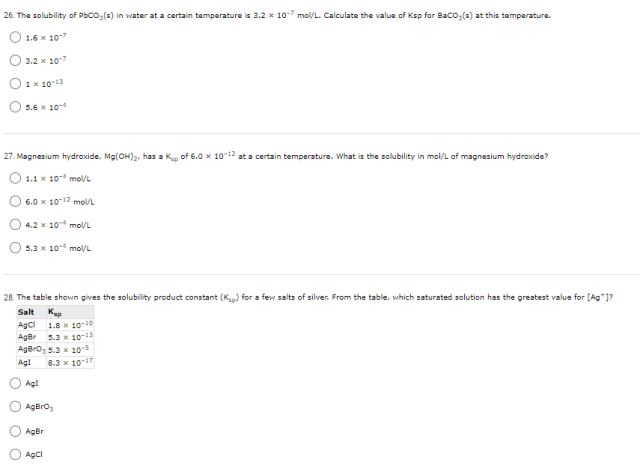
26.
PbCO₃(s) ⇌ Pb²⁺(aq) + CO₃²⁻(aq)
At equilibrium:
[Pb²⁺] = [CO₃²⁻] = 3.2 × 10⁻⁷
Ksp = [Pb²⁺] [CO₃²⁻] = (3.2 × 10⁻⁷)² = 1 × 10⁻¹³
The answer: 1 × 10⁻¹³
(the 3rd option)
====
27.
Mg(OH)₂(s) ⇌ Mg²⁺(aq) + 2OH⁻(aq)
At equilibrium:
[Mg²⁺] = s mol/L
[OH⁻] = 2s mol/L
Ksp = [Mg²⁺] [OH⁻]²
s (2s)² = 6.0 × 10⁻¹²
4s³ = 6.0 × 10⁻¹²
s = ³√(6.0 × 10⁻¹² / 4) = 1.1 × 10⁻⁴
The answer: 1.1 × 10⁻⁴ mol/L
(the 1st option)
====
28.
All of the four salts has AB formula type. Hence, the greater the Ksp, the greater the solubility.
AgBrO₃ has the greatest Ksp and thus the greatest solubility among the four salt. Hence, the saturated solution of AgBrO₃ has the greatest value for [Ag⁺].
The answer: AgBrO₃
(The second option)
PbCO₃(s) ⇌ Pb²⁺(aq) + CO₃²⁻(aq)
At equilibrium:
[Pb²⁺] = [CO₃²⁻] = 3.2 × 10⁻⁷
Ksp = [Pb²⁺] [CO₃²⁻] = (3.2 × 10⁻⁷)² = 1 × 10⁻¹³
The answer: 1 × 10⁻¹³
(the 3rd option)
====
27.
Mg(OH)₂(s) ⇌ Mg²⁺(aq) + 2OH⁻(aq)
At equilibrium:
[Mg²⁺] = s mol/L
[OH⁻] = 2s mol/L
Ksp = [Mg²⁺] [OH⁻]²
s (2s)² = 6.0 × 10⁻¹²
4s³ = 6.0 × 10⁻¹²
s = ³√(6.0 × 10⁻¹² / 4) = 1.1 × 10⁻⁴
The answer: 1.1 × 10⁻⁴ mol/L
(the 1st option)
====
28.
All of the four salts has AB formula type. Hence, the greater the Ksp, the greater the solubility.
AgBrO₃ has the greatest Ksp and thus the greatest solubility among the four salt. Hence, the saturated solution of AgBrO₃ has the greatest value for [Ag⁺].
The answer: AgBrO₃
(The second option)
2020-09-10 1:44 am
26. Bad question. You can calculate the Ksp of PbCO3, but not BaCO3.
PbCO3(s) <--> Pb2+(aq) + CO32-(aq)
From the molar solubility, [Pb2+] = [CO32-] = 3.2X10^-7 M
Ksp = [Pb2+][CO32-]
Ksp = (3.2X10^-7)^2 = 1.0X10^-13
27. Mg(OH)2(s) <--> Mg2+(aq) + 2 OH-(aq)
Ksp = [Mg2+] [OH-]^2 = 6.0X10^-12
Let solubility = x. Then, in a saturated solution, [Mg2+] = x and [OH-] = 2x. So,
Ksp = (x) (2x)^2 = 6.0X10^-12
4x^3 = 6.0X10^-12
x = 1.1X10^-4 M
28. The compound with the largest Ksp will be the most soluble, and so will have the highest [Ag+].
PbCO3(s) <--> Pb2+(aq) + CO32-(aq)
From the molar solubility, [Pb2+] = [CO32-] = 3.2X10^-7 M
Ksp = [Pb2+][CO32-]
Ksp = (3.2X10^-7)^2 = 1.0X10^-13
27. Mg(OH)2(s) <--> Mg2+(aq) + 2 OH-(aq)
Ksp = [Mg2+] [OH-]^2 = 6.0X10^-12
Let solubility = x. Then, in a saturated solution, [Mg2+] = x and [OH-] = 2x. So,
Ksp = (x) (2x)^2 = 6.0X10^-12
4x^3 = 6.0X10^-12
x = 1.1X10^-4 M
28. The compound with the largest Ksp will be the most soluble, and so will have the highest [Ag+].
收錄日期: 2021-05-01 22:39:44
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200909165707AACoWfw
