The answer is 2.53x10^-5
Anyone knows how to solve it?
Thanks
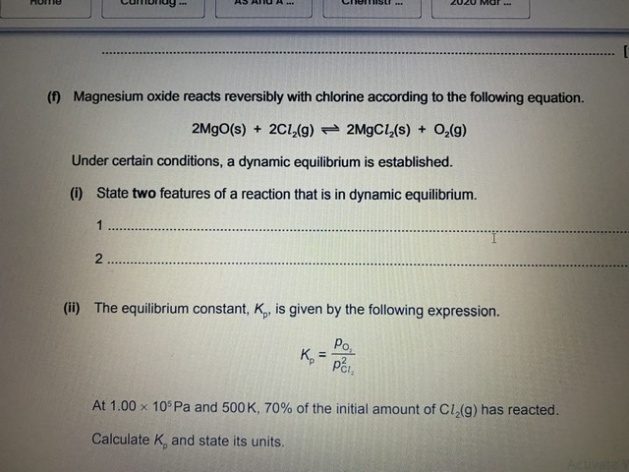
Calculate Kp?
2020-09-09 2:15 pm
回答 (1)
2020-09-09 3:35 pm
✔ 最佳答案
In the heterogeneous equilibrium, only the partial pressures of the gaseous components are involved in the equilibrium expression.2 MgO(s) + 2Cl₂(g) ⇌ 2MgCl₂(s) + O₂(g)
Initial: 1 mol 0 mol
Change: -0.3 mol +0.15 mol
Equilibrium: 0.7 mol 0.15 mol
Partial pressure of Cl₂, 𝑃Cl₂ = (1.00 × 10⁵ Pa) × [0.7/(0.7 + 0.15)]= 82350 Pa
Partial pressure of O₂, 𝑃O₂ = (1.00 × 10⁵ Pa) × [0.15/(0.7 + 0.15)] = 17650 Pa
Kp = 𝑃O₂/𝑃Cl₂² = (17650 Pa) / (82350 Pa)² = 2.60 × 10⁻⁵ Pa⁻¹
收錄日期: 2021-04-12 12:51:14
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200909061538AA5OTlR
