Please help?
2020-09-04 1:34 pm
回答 (1)
2020-09-04 3:51 pm
Refer to: https://en.wikipedia.org/wiki/Glutaric_acid
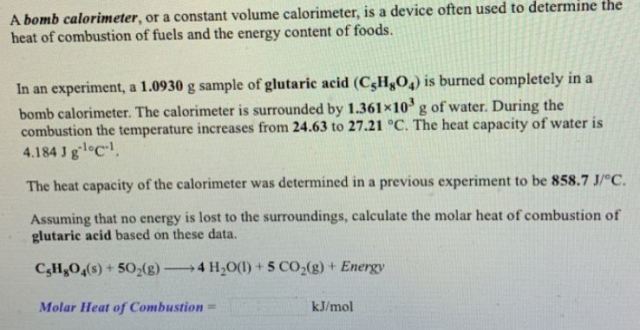
Molar mass of C₅H₈O₄ = 132.12 g/mol
No. of moles of C₅H₈O₄ = (1.0930 g) / (132.12 g/mol) = 8.2728 × 10⁻³ mol
Heat released in the reaction
= (Heat absorbed by the water) + (Heat absorbed by the calorimeter)
= [(1.361 × 10³) × 4.184 × (27.12 - 24.63)] + [858.7 × (27.12 - 24.63)] J
= 16317 J
= 16.317 kJ
As heat is given off in the combustion, the heat of combustion is negative.
The heat of combustion of C₅H₈O₄
= -(16.317 kJ) / (8.2728 × 10⁻³ mol)
= -1972 kJ/mol
Molar mass of C₅H₈O₄ = 132.12 g/mol
No. of moles of C₅H₈O₄ = (1.0930 g) / (132.12 g/mol) = 8.2728 × 10⁻³ mol
Heat released in the reaction
= (Heat absorbed by the water) + (Heat absorbed by the calorimeter)
= [(1.361 × 10³) × 4.184 × (27.12 - 24.63)] + [858.7 × (27.12 - 24.63)] J
= 16317 J
= 16.317 kJ
As heat is given off in the combustion, the heat of combustion is negative.
The heat of combustion of C₅H₈O₄
= -(16.317 kJ) / (8.2728 × 10⁻³ mol)
= -1972 kJ/mol
收錄日期: 2021-04-24 07:58:47
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200904053421AA7AtMV
