Enthalpy Change For The Forward Reaction?
2020-05-31 3:00 pm
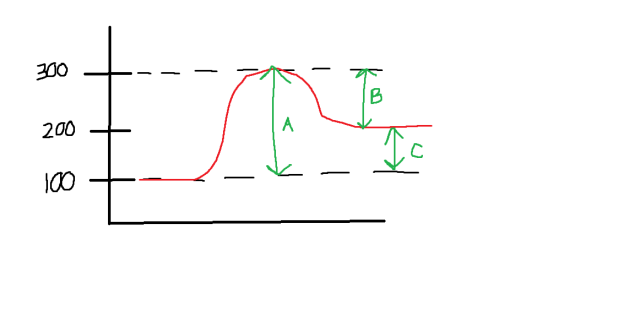
I am having trouble finding the enthalpy change for the forward reaction. I keep getting 100 kJ, but that isn't the right answer.
回答 (1)
2020-05-31 3:16 pm
Enthalpy change of the forward reaction
= (P.E. of products) - (P.E. of reactants)
= (200 - 100) kJ
= +100 kJ
= (P.E. of products) - (P.E. of reactants)
= (200 - 100) kJ
= +100 kJ
收錄日期: 2021-05-01 09:36:14
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200531070026AAHmrli
