a. IF5
b. PH3
c. CS2-2
d. CO3-2
e. CH4
which molecule or ion below has a lewis structure that does not obey the octet rule?
2020-05-16 8:39 pm
回答 (2)
2020-05-17 12:28 am
The option c should be CS₂ instead.
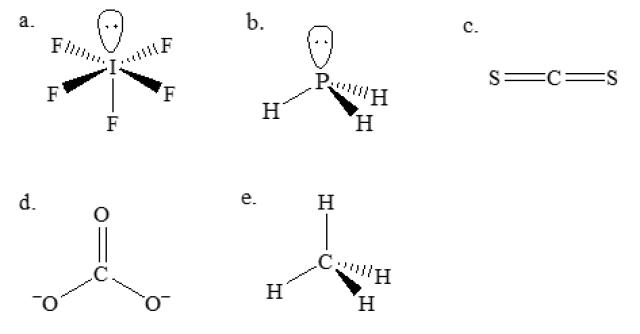
The structures of the options are shown below.
For IF₅ (a), the central I atom has 5 I-F single bonds and 1 lone pair of electrons. There are 6 × 2 = 12 electrons in the outermost shell of the central I atom, and thus it does not obey octet rule.
For PH₃ (b), the central P atom has 3 P-H single bonds and 1 lone pair of electrons. There are 4 × 2 = 8 electrons in the outermost shell of the central P atom, and thus it obeys octet rule.
For CS₂ (c), the central C atom has 2 C=S double bonds. There are 4 × 2 = 8 electrons in the outermost shell of the central C atom, and thus it obeys octet rule.
For CO₃²⁻ (d), the central C atom has 1 C=O double bond and 2 C-O single bonds. There are 4 × 2 = 8 electrons in the outermost shell of the central C atom, and thus it obeys octet rule.
For CH₄ (e), the central C atom has 4 C-H single bonds. There are 4 × 2 = 8 electrons in the outermost shell of the central C atom, and thus it obeys octet rule.
The answer: a. IF₅
====
A simple short-cut to the answer:
In IF₅, the central atom has at least 10 electrons in the outermost shell (at least 5 I-F bonds).
The answer: a. IF₅
The structures of the options are shown below.
For IF₅ (a), the central I atom has 5 I-F single bonds and 1 lone pair of electrons. There are 6 × 2 = 12 electrons in the outermost shell of the central I atom, and thus it does not obey octet rule.
For PH₃ (b), the central P atom has 3 P-H single bonds and 1 lone pair of electrons. There are 4 × 2 = 8 electrons in the outermost shell of the central P atom, and thus it obeys octet rule.
For CS₂ (c), the central C atom has 2 C=S double bonds. There are 4 × 2 = 8 electrons in the outermost shell of the central C atom, and thus it obeys octet rule.
For CO₃²⁻ (d), the central C atom has 1 C=O double bond and 2 C-O single bonds. There are 4 × 2 = 8 electrons in the outermost shell of the central C atom, and thus it obeys octet rule.
For CH₄ (e), the central C atom has 4 C-H single bonds. There are 4 × 2 = 8 electrons in the outermost shell of the central C atom, and thus it obeys octet rule.
The answer: a. IF₅
====
A simple short-cut to the answer:
In IF₅, the central atom has at least 10 electrons in the outermost shell (at least 5 I-F bonds).
The answer: a. IF₅
2020-05-16 9:38 pm
a. IF5 <--- for sure
c. CS2-2 <--- I have not heard of this ion. If it exists, it would not obey the octet rule.
c. CS2-2 <--- I have not heard of this ion. If it exists, it would not obey the octet rule.
收錄日期: 2021-04-18 18:33:08
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200516123959AAx3uwY
