What is the rate equation for the reaction?
a. Rate = k (PNO) 2 (PH2)
b. Rate = k (PNO) 2 (PH2) 2
c. Rate = k (PNO) (PH2)
d. Rate = k (PNO) (PH2) 2
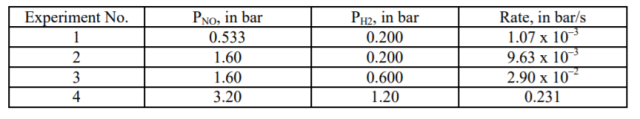
The following data were collected for the reaction 2 NO(g) + 2 H2(g) ? N2(g) + 2 H2O (l), at 826°C and with constant volume.?
2020-05-15 1:44 pm
回答 (1)
2020-05-15 2:03 pm
✔ 最佳答案
2NO(g) + 2H₂(g) → N₂(g) + 2H₂O(ℓ)Rate = k (PNO)ˣ (PH₂)ʸ
Consider Experiments 1 and 2. When PH₂ is kept constant:
(9.63 × 10³)/(1.07 × 10⁻³) = (1.60/0.533)ˣ
9 = 3ˣ
3ˣ = 3²
x = 2
Consider Experiments 2 and 3. When PNO is kept constant:
(2.90 × 10⁻²)/(9.63 × 10⁻³) = (0.6/0.2)ʸ
3 = 3ʸ
3ʸ = 3¹
y = 1
The answer: a. Rate = k (PNO)² (PH₂)
收錄日期: 2021-04-30 17:15:40
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200515054417AAvLtsB
