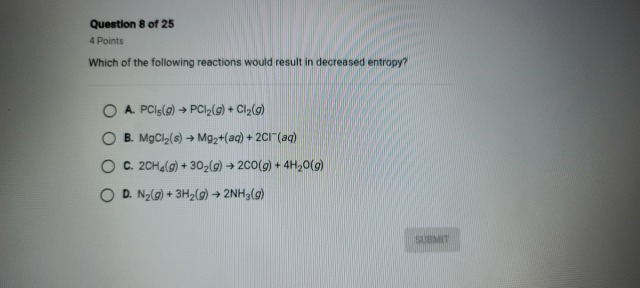
Which of the following reactions would result in decreased entropy?
2020-05-12 1:12 pm
回答 (2)
2020-05-12 5:21 pm
Entropy is a measure of degree of randomness. The higher the degree of randomness, the greater the entropy is.
A. False
The degree of randomness of the system increases when 1 mole of gaseous molecules is converted to 2 moles of gaseous molecules. Hence, entropy increases.
B. False
In solid state, Mg²⁺ and Cl⁻ ions are well arranged in pattern. When MgCl₂ dissolves, the ions become mobile and thus the degree of randomness of the system increases. Hence, entropy increases.
C. False
The degree of randomness of the system increases when 5 moles of gaseous molecules are converted to 6 moles of gaseous molecules. Hence, entropy increases.
D. True
The degree of randomness of the system decreases when 4 moles of gaseous molecules are converted to 2 moles of gaseous molecules. Hence, entropy decreases.
The answer: D. N₂(g) + 3H₂(g) → 2NH₃(g)
A. False
The degree of randomness of the system increases when 1 mole of gaseous molecules is converted to 2 moles of gaseous molecules. Hence, entropy increases.
B. False
In solid state, Mg²⁺ and Cl⁻ ions are well arranged in pattern. When MgCl₂ dissolves, the ions become mobile and thus the degree of randomness of the system increases. Hence, entropy increases.
C. False
The degree of randomness of the system increases when 5 moles of gaseous molecules are converted to 6 moles of gaseous molecules. Hence, entropy increases.
D. True
The degree of randomness of the system decreases when 4 moles of gaseous molecules are converted to 2 moles of gaseous molecules. Hence, entropy decreases.
The answer: D. N₂(g) + 3H₂(g) → 2NH₃(g)
2020-05-12 1:29 pm
D. results in decreased entropy.
收錄日期: 2021-05-01 01:05:04
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200512051256AAq9IBn


