Please help chem pic?
2020-04-27 12:17 pm
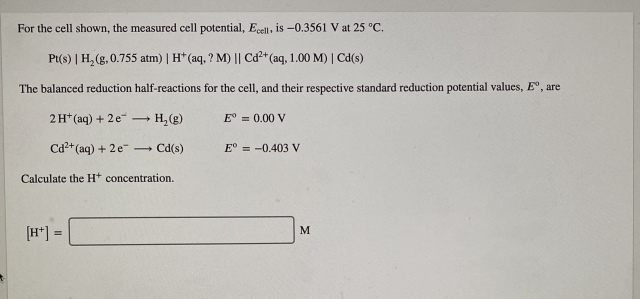
回答 (1)
2020-04-27 6:28 pm
✔ 最佳答案
Overall reaction:H₂(g) + Cd²⁺(aq) → 2H⁺(aq) + Cd(s)
E°(cell)
= E°(red) - E°(oxid)
= (-0.403) - (0.00) V
= -0.403 V
Nernst equation:
E(cell) = E°(cell) - {RT/(nF)} ln{[H⁺]²/(P(H₂)•[Cd²⁺]
-0.3561 = -0.403 - {8.314 × 298 /(2 × 96485)} ln{[H⁺]²/(0.755 × 1)}
0.01284 ln[H⁺]² - 0.01284 ln(0.755) = -0.403 + 0.3561
0.02568 ln[H⁺] = -0.05051
ln[H⁺] = -1.967
[H⁺] = e⁻¹˙⁹⁶⁷
[H⁺] = 0.140M
收錄日期: 2021-05-01 22:36:02
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200427041714AAp6dY2
