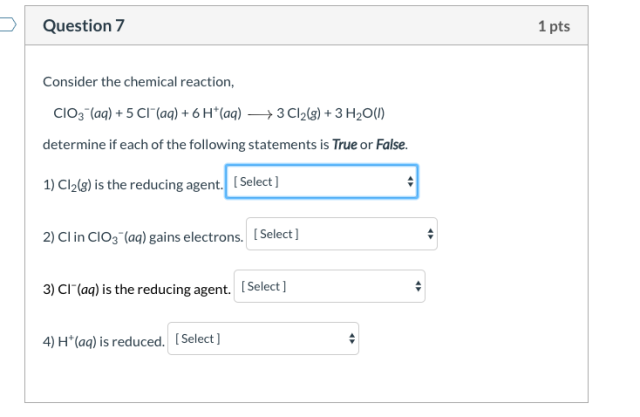
Consider ClO3−(aq) + 5 Cl−(aq) + 6 H+(aq) ⟶ 3 Cl2(g) + 3 H2O(l) determine if each of the following statements is True or False. ?
2020-04-20 5:51 pm
回答 (1)
2020-04-20 6:33 pm
✔ 最佳答案
1) FalseCl₂ is a product. We can classify the reactants of a redox reaction to be an oxidizing agent or a reducing agent, but not the products.
2) True
2ClO₃⁻(aq) + 12H⁺(aq) + 10e⁻ → Cl₂(g) + 6H₂O(ℓ)
3) True
2Cl⁻(aq) → Cl₂(g) + 2e⁻
Cl⁻ is oxidized because its oxidation number increases from -1 to 0.
Hence, it is a reducing agent.
4) False
The oxidation number of H in H⁺ = +1
The oxidation number of H in H₂O = +1
The oxidation number of H is unchanged in the reaction. Hence, H⁺ is neither reduced nor oxidized.
收錄日期: 2021-05-01 09:40:26
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200420095105AAsLtfo
