I've attached a picture of the question below.
When Ca(IO3)2 dissolves in a solution containing H+, the following two reactions occur:Ca(IO3)2 <=> Ca2+ + 2 IO3-Reaction 1H+ + IO3- <=> HIO3 Reaction 2.The answer provided by the book says that A is the correct answer as it is "purely definitional; products over reactants". However, if that is the case.. wouldn't the correct answer be D? I had D as my answer but not sure whether the answer stated as A was a mistake by the author, as after all the book does contain a few errors.
Rate of reaction, constant question?
2020-04-14 10:24 pm
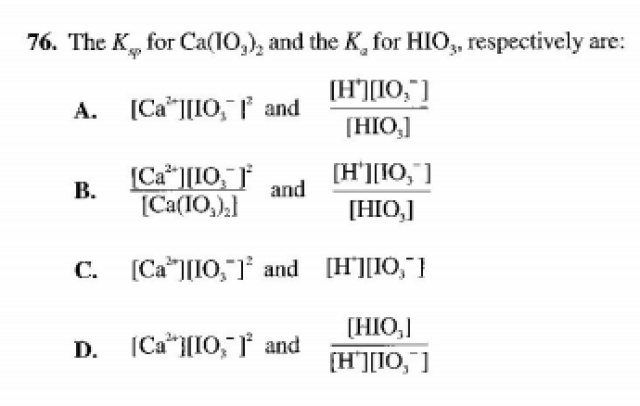
回答 (2)
2020-04-14 11:03 pm
✔ 最佳答案
The balanced equation for the solubilizing of Ca(IO₃)₂:Ca(IO₃)₂(s) ⇌ Ca²⁺(aq) + 2IO₃⁻(aq) …… Ksp
Ksp = [Ca²⁺] [IO₃⁻]²
The balanced equation for dissociation (ionization) of HIO₃:
HIO₃(aq) ⇌ H⁺(aq) + IO₃⁻(aq) …… Ka
Ka = [H⁺] [IO₃⁻] / [HIO₃]
The answer: A. [Ca²⁺] [IO₃⁻]² and [H⁺] [IO₃⁻] / [HIO₃]
2020-04-14 11:57 pm
the question has 2 parts..
.. Ksp for Ca(IO3)2 dissolving in water
and
.. Ka for HIO3
read it closely.. .
.. Ksp & Ka
for a salt dissolving
.. XY ----> X(+) + Y(-)
Ksp is defined as
.. Ksp = [X(+)] * [Y(-)]
for an acid
.. HA ----> H(+) + A(-)
Ka is defined as
.. Ka = ([H(+)] + [A(-)]) / [HA]
.. Ksp for Ca(IO3)2 dissolving in water
and
.. Ka for HIO3
read it closely.. .
.. Ksp & Ka
for a salt dissolving
.. XY ----> X(+) + Y(-)
Ksp is defined as
.. Ksp = [X(+)] * [Y(-)]
for an acid
.. HA ----> H(+) + A(-)
Ka is defined as
.. Ka = ([H(+)] + [A(-)]) / [HA]
收錄日期: 2021-04-24 07:50:09
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200414142429AACeHeQ
