Physics problem: how to do, thanks?
2020-04-14 7:36 pm
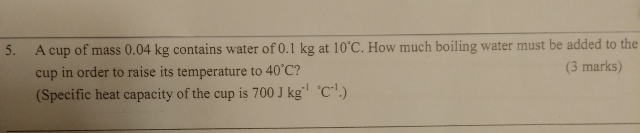
回答 (1)
2020-04-14 10:55 pm
✔ 最佳答案
Let m - mass of boiling water to be addedspecific heat capacity of water=4.2 J kg⁻¹ ⁰C⁻¹
heat lost by boiling water = heat gained by cold water + heat gained by cup
m×4.2×(100-40)=0.1×4.2×(40-10)+0.04×700×(40-10)
252m=12.6+840
∴ m=3.38 kg (to 2 dec.pl.)
收錄日期: 2021-04-24 07:51:17
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200414113609AACBe2g

