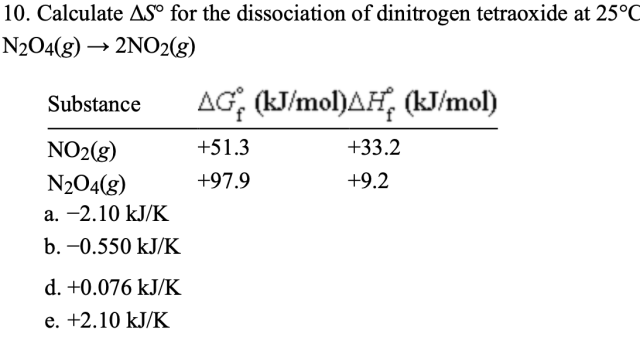
Calculate ΔS° for the dissociation of dinitrogen tetraoxide at 25°C?
2020-04-11 9:24 pm
回答 (2)
2020-04-12 12:37 am
N₂O₄(g) → 2NO₂(g)
ΔG° = 2 ΔG°f[NO₂(g)] - ΔG°f[N₂O₄(g)]
= 2 (+51.3) - (+97.9) kJ
= +4.7 kJ
= +4700 J
ΔG° = 2 ΔG°f[NO₂(g)] - ΔG°f[N₂O₄(g)]
ΔG° = 2 (+51.3) - (+97.9) kJ
ΔG° = +4.7 kJ
ΔH° = 2 ΔH°f[NO₂(g)] - ΔH°f[N₂O₄(g)]
ΔH° = 2 (+33.2) - (+9.2) kJ
ΔH° = +57.2 kJ
T = (273 + 25) K
T = 298 K
ΔG° = ΔH° - TΔS°
(+4.7 kJ) = (+57.2 kJ) - (298 K) ΔS°
ΔS° = (+57.2 - 4.7) / 298 kJ/K
ΔS° = +0.176 kJ/K
The answer: None of the 4 options
ΔG° = 2 ΔG°f[NO₂(g)] - ΔG°f[N₂O₄(g)]
= 2 (+51.3) - (+97.9) kJ
= +4.7 kJ
= +4700 J
ΔG° = 2 ΔG°f[NO₂(g)] - ΔG°f[N₂O₄(g)]
ΔG° = 2 (+51.3) - (+97.9) kJ
ΔG° = +4.7 kJ
ΔH° = 2 ΔH°f[NO₂(g)] - ΔH°f[N₂O₄(g)]
ΔH° = 2 (+33.2) - (+9.2) kJ
ΔH° = +57.2 kJ
T = (273 + 25) K
T = 298 K
ΔG° = ΔH° - TΔS°
(+4.7 kJ) = (+57.2 kJ) - (298 K) ΔS°
ΔS° = (+57.2 - 4.7) / 298 kJ/K
ΔS° = +0.176 kJ/K
The answer: None of the 4 options
2020-04-12 12:28 am
ΔG°rxn = 2(51.3) - 97.9 = +4.7 kJ/mol
ΔH°rxn = 2(33.2) - 9.2 = 57.2 kJ/mol
ΔG°rxn = ΔH°rxn - TΔS°rxn
4.7 kJ/mol = 57.2 kJ/mol - 298 K ΔS°rxn
ΔS°rxn = 0.176 kJ/K = 176 J/K
I'm not getting any of the answers you've shown, but option c. is not shown. Is c. possible +0.176 kJ/K?
ΔH°rxn = 2(33.2) - 9.2 = 57.2 kJ/mol
ΔG°rxn = ΔH°rxn - TΔS°rxn
4.7 kJ/mol = 57.2 kJ/mol - 298 K ΔS°rxn
ΔS°rxn = 0.176 kJ/K = 176 J/K
I'm not getting any of the answers you've shown, but option c. is not shown. Is c. possible +0.176 kJ/K?
收錄日期: 2021-05-02 11:16:23
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200411132442AAdyV3m
