calculate standard enthalpy change for reaction?
2020-04-07 1:48 pm
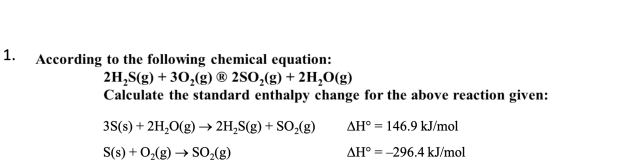
回答 (3)
2020-04-07 2:10 pm
✔ 最佳答案
You need "2 H2S(g)" on the left. The only given equation that mentions H2S(g) is the first one, so write the first given equation backwards:2 H2S(g) + SO2(g) → 3 S(s) + 2 H2O(g), ΔH = −146.9 kJ
You also need "3 O2(g)" on the left. The only given equation that mentions O2(g) is the second one, so multiply the second given equation by 3:
3 S(s) + 3 O2(g) → 3 SO2(g), ΔH = −889.2 kJ
Add the two equations here:
2 H2S(g) + SO2(g) + 3 S(s) + 3 O2(g) →3 S(s) + 2 H2O(g) + 3 SO2(g),
ΔH = −146.9 kJ −889.2 kJ
Cancel like amounts on opposite sides of the arrow, and do the arithmetic for ΔH:
2 H2S(g) + 3 O2(g) → 2 H2O(g) + 2 SO2(g), ΔH = −1036.1 kJ
2020-04-07 2:39 pm
Rewrite the two thermochemical equations as follows:
2H₂S(g) + SO₂(g) → 3S(s) + 2H₂O(g) … ΔH°₁ = -146.9 kJ/mol
3S(s) + 3O₂(g) → 3SO₂(g) … ΔH°₂ = 3(-296.4) = -889.2 kJ/mol
Add the above two thermochemical equations, and cancel 3S(s) and SO₂(g) on each side:
2H₂S(g) + 3O₂(g) → 2SO₂(g) + 2H₂O(g) … ΔH°
ΔH° = ΔH°₁ + ΔH°₂ = (-146.9) + (-889.2) = -1036.1 kJ/mol
2H₂S(g) + SO₂(g) → 3S(s) + 2H₂O(g) … ΔH°₁ = -146.9 kJ/mol
3S(s) + 3O₂(g) → 3SO₂(g) … ΔH°₂ = 3(-296.4) = -889.2 kJ/mol
Add the above two thermochemical equations, and cancel 3S(s) and SO₂(g) on each side:
2H₂S(g) + 3O₂(g) → 2SO₂(g) + 2H₂O(g) … ΔH°
ΔH° = ΔH°₁ + ΔH°₂ = (-146.9) + (-889.2) = -1036.1 kJ/mol
2020-04-07 1:49 pm
Anon don't get no love....
收錄日期: 2021-04-18 18:26:29
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200407054807AAo5TM8

