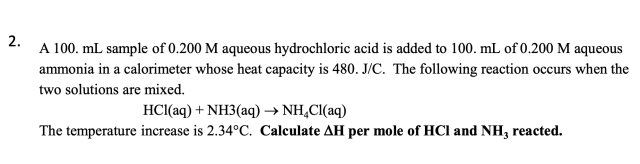
Mole of HCl and NH3. Reacted Chemistry ?
2020-04-07 1:45 pm
回答 (2)
2020-04-07 2:22 pm
Moles of NH₃ reacted = Moles of HCl reacted = (0.200 mol/L) × (100/1000 L) = 0.0200 mol
Heat absorbed by the calorimeter = (480 J/°C) × (2.34°C) = 1123.2 J
Heat given off in the reaction = 1123.2 J
As heat is given off in the reaction, the reaction is exothermic with ΔH < 0
ΔH = -(1123.2 J) / (0.0200 mol) = -56200 J/mol = -56.2 kJ/mol
Heat absorbed by the calorimeter = (480 J/°C) × (2.34°C) = 1123.2 J
Heat given off in the reaction = 1123.2 J
As heat is given off in the reaction, the reaction is exothermic with ΔH < 0
ΔH = -(1123.2 J) / (0.0200 mol) = -56200 J/mol = -56.2 kJ/mol
2020-04-07 2:15 pm
HCl and NH3 are present in equimolar amounts, so there is no excess.
(480 J/°C) x (2.34°C) = 1123.2 J produced
(1123.2 J) / ((0.100 L) x (0.200 mol/L)) = 56160 J / mol = 56.2 kJ/mol
The "mol" at the end of the last line can be either HCl, or NH3, or NH4Cl as you wish.
(480 J/°C) x (2.34°C) = 1123.2 J produced
(1123.2 J) / ((0.100 L) x (0.200 mol/L)) = 56160 J / mol = 56.2 kJ/mol
The "mol" at the end of the last line can be either HCl, or NH3, or NH4Cl as you wish.
收錄日期: 2021-04-18 18:27:11
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200407054557AAyQacb

