Chemistry: E (0) From Reduction?
2020-04-06 10:06 am
Please help!!
回答 (1)
2020-04-06 11:04 am
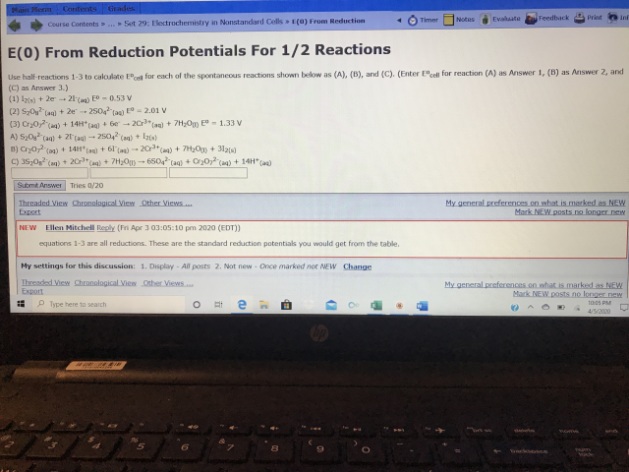
A)
E°(cell)
= E°(reduction) - E°(oxidation)
= E°(S₂O₈²⁻|SO₄²⁻) - E°(I₂|I⁻)
= (2.01 - 0.53) V
= 1.48 V
B)
E°(cell)
= E°(reduction) - E°(oxidation)
= E°(Cr₂O₇²⁻,H⁺|Cr³⁺) - E°(I₂|I⁻)
= (1.33 - 0.53) V
= 0.80 V
C)
E°(cell)
= E°(reduction) - E°(oxidation)
= E°(S₂O₈²⁻|SO₄²⁻) - E°(Cr₂O₇²⁻,H⁺|Cr³⁺)
= (2.01 - 1.33) V
= 0.68 V
E°(cell)
= E°(reduction) - E°(oxidation)
= E°(S₂O₈²⁻|SO₄²⁻) - E°(I₂|I⁻)
= (2.01 - 0.53) V
= 1.48 V
B)
E°(cell)
= E°(reduction) - E°(oxidation)
= E°(Cr₂O₇²⁻,H⁺|Cr³⁺) - E°(I₂|I⁻)
= (1.33 - 0.53) V
= 0.80 V
C)
E°(cell)
= E°(reduction) - E°(oxidation)
= E°(S₂O₈²⁻|SO₄²⁻) - E°(Cr₂O₇²⁻,H⁺|Cr³⁺)
= (2.01 - 1.33) V
= 0.68 V
收錄日期: 2021-04-18 18:27:44
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200406020612AACLF0o
