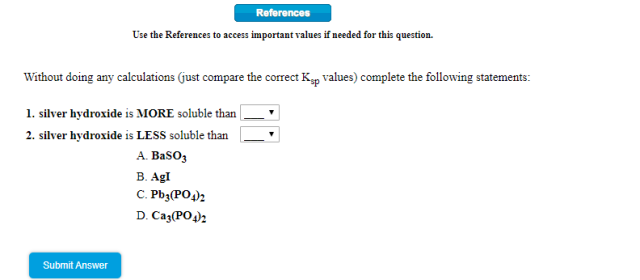
1) silver hydroxide is MORE soluble than
2) silver hydroxide is LESS soluble than
chem help pls ?
2020-04-03 12:24 pm
回答 (2)
2020-04-03 1:49 pm
✔ 最佳答案
The formula of AgOH is AB type. We just consider the options with AB type formula, i.e. BaSO₃ and AgI.Ksp(AgOH) = 2.0 × 10⁻⁸
Ksp(BaSO₃) = 8 × 10⁻⁷
Ksp(AgI) = 8.5 × 10⁻¹⁷
Ksp(BaSO₃) > Ksp(AgOH) > Ksp(AgI)
Hence, solubility: BaSO₃ > AgOH) > AgI
1. Silver hydroxide is MORE soluble than __B__.
2. Silver hydroxide is LESS soluble than __A__.
2020-04-03 1:11 pm
And where are these "correct Ksp values"?
收錄日期: 2021-04-18 18:31:17
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200403042427AAjjqMa

