Chemistry problem: how to do, thanks.?
2020-03-25 8:56 pm
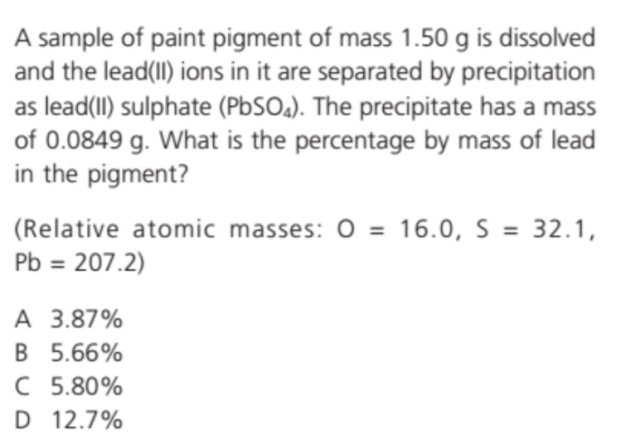
回答 (1)
2020-03-25 11:23 pm
✔ 最佳答案
molecular mass of PbSO₄=207.2+32.1+16(4)=303.3mass of lead in precipitate(PbSO₄)
207.2
= 0.0849 ×--------- = 0.0580
303.3
%tage of mass in Pb pigment
0.0580
= ---------- × 100% = 3.87% (to 3 sig. fig.) - - - - (A)
1.50
收錄日期: 2021-04-24 07:50:14
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20200325125637AAlw1yN

