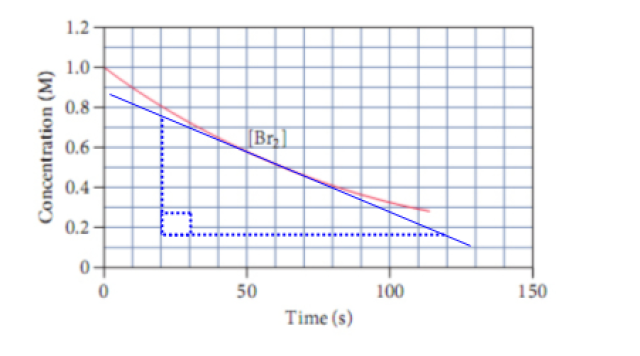
Calculate the avg reaction rate b/w 0-25s
-I got 0.010M/s
Calculate the instantaneous rate of formation of HBr at 50 s?
the answer is 0.012M/s but how do you get that??
更新1:
****I should add that it should be done WITHOUT using Calculus since this is a class that doesn't require it as a prerequisite*****