What is the ranking of the following compounds (1 = strongest) on the strength of their intermolecular forces between molecules? And why?
2019-02-13 5:55 pm
回答 (1)
2019-02-13 6:53 pm
✔ 最佳答案
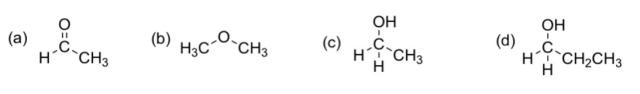
1. (d) CH₃CH₂CH₂OH 2. (c) CH₃CH₂OH
3. (a) CH₃CHO
4. (b) CH₃OCH₃
All the four compounds are small polar molecules.
In (c) and (d), O is a very electronegative atom and thus H is rather bare in electrons opposite the O-H bond. Hence, the intermolecular forces between their molecules are mainly intermolecular hydrogen bonds. In (a) and (b), there are no hydrogen bonds and the polar molecules are held together by mainly dipole-dipole attractions. For small molecules, intermolecular hydrogen bonds are strong than dipole-dipole attractions. Therefore,
Intermolecular forces: (c)/(d) > (a)/(b)
Compare (a) and (b). Since C=O bond is much polar than C-O bond, CH₃CHO is polar than CH₃OCH₃. The more polar the small molecules, the stronger intermolecular forces between them are. Therefore,
Intermolecular forces: (a) > (b)
Compare (c) and (b). Both of them have intermolecular hydrogen bonds. However, CH₃CH₂CH₂OH molecule has a longer hydrocarbon chain than CH₃CH₂OH, and thus CH₃CH₂CH₂OH molecules have stronger van der Waals' and thus have stronger intermolecular forces. Therefore,
Intermolecular forces: (d) > (c)
Conclusively, intermolecular forces: (d) > (c) > (a) > (b)
1. (d) CH₃CH₂CH₂OH
2. (c) CH₃CH₂OH
3. (a) CH₃CHO
4. (b) CH₃OCH₃
All the four compounds are small polar molecules.
In (c) and (d), O is a very electronegative atom and thus H is rather bare in electrons opposite the O-H bond. Hence, the intermolecular forces between their molecules are mainly intermolecular hydrogen bonds. In (a) and (b), there are no hydrogen bonds and the polar molecules are held together by mainly dipole-dipole attractions. For small molecules, intermolecular hydrogen bonds are strong than dipole-dipole attractions. Therefore,
Intermolecular forces: (c)/(d) > (a)/(b)
Compare (a) and (b). Since C=O bond is much polar than C-O bond, CH₃CHO is polar than CH₃OCH₃. The more polar the small molecules, the stronger intermolecular forces between them are. Therefore,
Intermolecular forces: (a) > (b)
Compare (c) and (b). Both of them have intermolecular hydrogen bonds. However, CH₃CH₂CH₂OH molecule has a longer hydrocarbon chain than CH₃CH₂OH, and thus CH₃CH₂CH₂OH molecules have stronger van der Waals' and thus have stronger intermolecular forces. Therefore,
Intermolecular forces: (d) > (c)
Conclusively, intermolecular forces: (d) > (c) > (a) > (b)
This can be shown by their boilng points: (d) > (c) > (a) > (b)
收錄日期: 2021-04-24 01:16:56
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20190213095544AAhWxpt
