There are times when acid/base reaction is also a precipitation reaction. Give an example of two reactants you would combine that would?
2019-02-13 6:04 am
Give an example of two reactants you could combine that would produce such a result.
回答 (2)
2019-02-13 5:35 pm
Aqueous sulfuric acid is a strong acid, while aqueous barium hydroxide is a strong base. When they are mixed, neutralization occurs and water is formed. Simultaneously, a precipitate of barium sulfate is formed.
The equation is:
H₂SO₄(aq) + Ba(OH)₂(aq) → BaSO₄(s) + 2H₂O(l)
The equation is:
H₂SO₄(aq) + Ba(OH)₂(aq) → BaSO₄(s) + 2H₂O(l)
2019-02-13 12:06 pm
Sodium hydrogen phosphate and calcium nitrate would form a white precipitate. sodium carbonate and calcium nitrate would also form a precipitate.
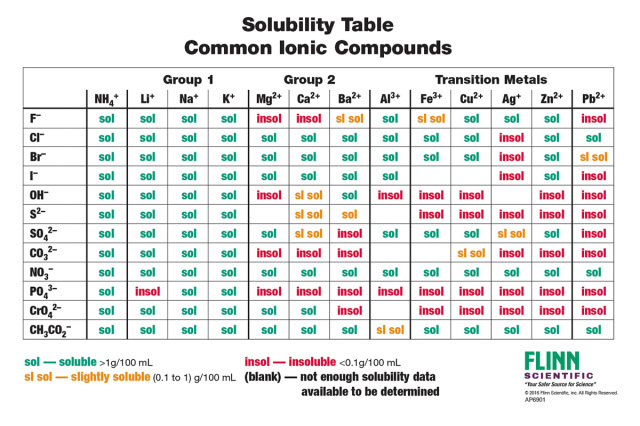
To know if a precipitate forms make sure to know your solubility rules.
To know if a precipitate forms make sure to know your solubility rules.
收錄日期: 2021-04-27 23:15:52
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20190212220408AAGioGP
