In the phase diagram, what is the temperature and pressure of the critical point?
https://api.agilixbuzz.com/Resz/~0.RyEM7a0dtX4KIoRE.oiZASQnTp1JJek-zaGym2fH4OA7Lvn658sbjGOB4PXo/55699901,9C0,0/Assets/Media/Images/Image8283.jpg
80°C and 7 atm
80°C and 2 atm
600°C and 12 atm- my answer
520°C and 7 atm
Please Help?
2018-08-25 9:54 am
回答 (3)
2018-08-25 12:41 pm
✔ 最佳答案
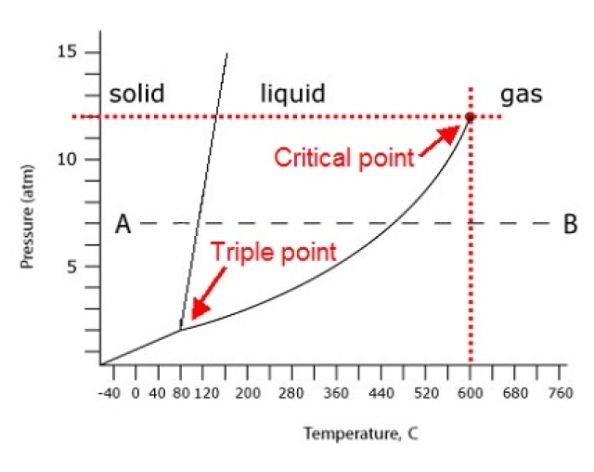
Critical pointThe critical point is where the liquid phase is no longer distinguishable from the gaseous phase. On your graph, the critical point is the dot between the words "liquid" and "gas."
Look at the graph below. .... you will see that at the critical point the pressure is 12 atm and the temperature is 600C.
.
2018-08-25 11:29 am
Refer to the diagram below.
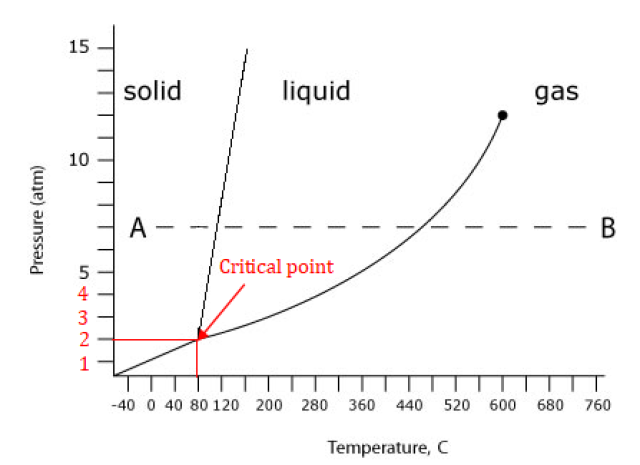
The vertical line passing through the critical point cuts the x-axis at 80°C, and the horizontal line passing through the critical point cuts the y-axis at 2 atm. Hence, the critical point is at 80°C and 2 atm.
The answer: 80°C and 2 atm
The vertical line passing through the critical point cuts the x-axis at 80°C, and the horizontal line passing through the critical point cuts the y-axis at 2 atm. Hence, the critical point is at 80°C and 2 atm.
The answer: 80°C and 2 atm
2018-08-25 10:15 am
correct !
收錄日期: 2021-04-24 01:12:57
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20180825015425AAQyvMU

