Please help me this Chemistry question. Thanks!?
2018-06-25 10:27 am
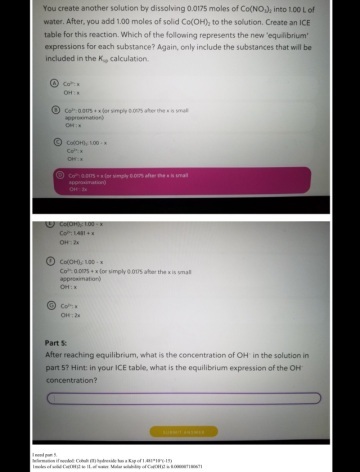
Part 5:
回答 (1)
2018-06-25 12:48 pm
✔ 最佳答案
Refer to: http://www4.ncsu.edu/~franzen/public_html/CH201/data/Solubility_Product_Constants.pdfKsp for Co(OH)₂ = 5.92 × 10⁻¹⁵
(The Ksp values from different sources may be slightly different.)
0.0175 mol Co(NO₃)₂ completely dissociates to give 0.0175 mol Co²⁺ ions.
Co(OH)₂ partly dissociates to give y mol Co²⁺ ions and 2y mol OH⁻ ions.
Consider the solubility equilibrium of Co(OH)₂.
__________ Co(OH)₂(s) ⇌ Co²⁺(aq) __ + __ 2OH⁻(aq) _____ Ksp = 5.92 × 10⁻¹⁵
Initial: ________________ 0.0175 M ________ 0 M
Change: _______________ +y mol _______ + 2y mol
Equilibrium: _________(0.0175 + y) M ______ 2y M
As Ksp is very small and due to the common ion effect in the presence of Co²⁺ ions, y ≪ 0.0175
Hence, it is assumed that [Co²⁺] at equilibrium = (0.0175 + y) M ≈ 0.0175 M
At equilibrium:
Ksp = 5.92 × 10⁻¹⁵
[Co²⁺] [OH⁻]² = 5.92 × 10⁻¹⁵
0.0175 × (2y)² = 5.92 × 10⁻¹⁵
0.07y² = 5.92 × 10⁻¹⁵
y = √(5.92 × 10⁻¹⁵ / 0.07) = 2.91 × 10⁻⁷
[OH⁻] at equilibrium = 2y M = 2 × (2.91 × 10⁻⁷) M = 5.82 × 10⁻⁷ M
收錄日期: 2021-04-24 01:05:04
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20180625022714AAbikZ4
