You may select more than one response.
(a) The central N atom is sp2 hybridized.
(b) The central N atom has a formal charge of +1.
(c) There are two σ and two π bonds.
(d) The structure of N2O is best represented as a hybrid of three equivalent resonance structures.
(e) The N2O molecule is linear.
(f) The two N atoms have the same oxidation state.
(g) The N-N-O angle is 60 degrees.
Which of the following statements concerning the N2O molecule is/are true? The atoms are bonded in the order NNO.?
2018-04-07 8:27 am
回答 (1)
2018-04-07 3:05 pm
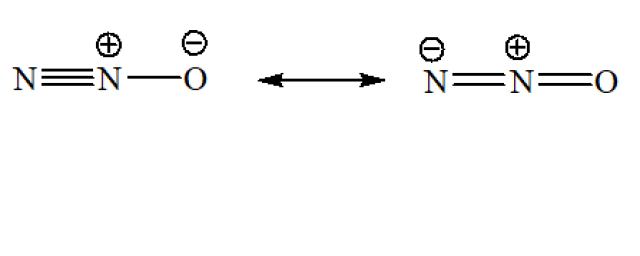
Refer to the resonance structures of N₂O which is linear in shape.
(a) false
The central N atom is sp hybridized because the molecule is linear.
(b) true
(c) true
(d) false
There are two resonance structures only.
(e) true
(f) false
The oxidation state of the terminal N atom is 0, while that of the central N atom is +2.
(g) false
It is 180°
The answers: (b), (c) and (e)
(a) false
The central N atom is sp hybridized because the molecule is linear.
(b) true
(c) true
(d) false
There are two resonance structures only.
(e) true
(f) false
The oxidation state of the terminal N atom is 0, while that of the central N atom is +2.
(g) false
It is 180°
The answers: (b), (c) and (e)
收錄日期: 2021-04-24 01:03:02
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20180407002729AAwEUQ1
