Enthalpy change___chemistry?
2018-03-22 4:48 pm
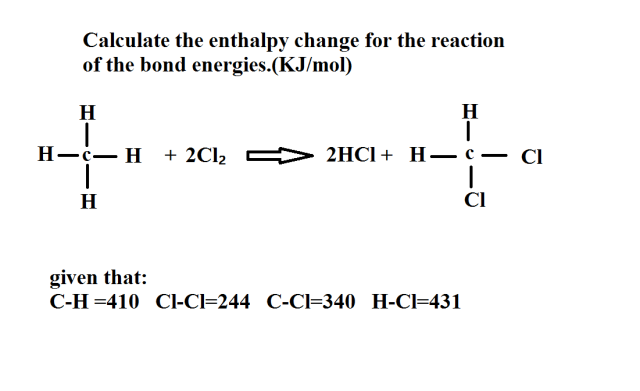
回答 (1)
2018-03-22 5:32 pm
✔ 最佳答案
Enthalpy change for bond breaking= +(E[C-H]×4 + E[Cl-Cl]×2)
= +(410×4 + 244×2) kJ
= +2128 kJ
Enthalpy change for bond formation
= -(E[H-Cl]×2 + E[C-H]×2 + E[C-Cl])
= -(431×2 + 410×2 + 340×2)
= -2362 kJ
Enthalpy change for the reaction, ΔH
= (+2128 - 2362) kJ
= -234 kJ
收錄日期: 2021-04-24 01:01:00
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20180322084836AAK5yVl
