How do glacial acetate acid, benzoic acid, and stearic acid soluble in ether?
2018-03-19 9:37 pm
回答 (1)
2018-03-20 12:43 am
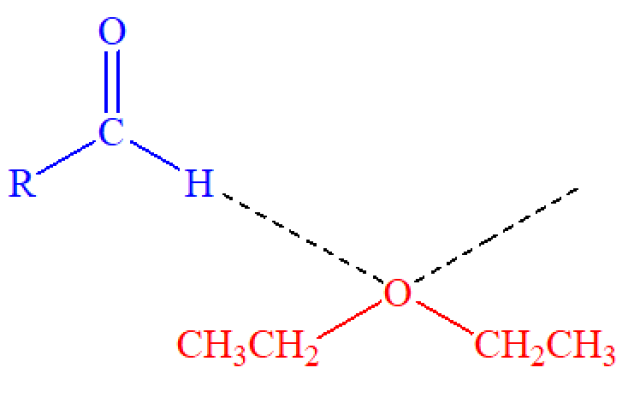
Some organic acids (denoted as R-COOH), such as glacial acetic acid, benzoic acid and stearic acid, are soluble in ether (CH₃CH₂OCH₂CH₃). This is due to the formation of hydrogen bonds between the acid molecules and ether molecules as shown in the following diagram, where the dot lines indicate hydrogen bonds.
收錄日期: 2021-04-24 01:00:16
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20180319133753AAoJFx8
