how many bonding and lone pair does cf4 have?
2018-03-11 5:26 pm
回答 (1)
2018-03-11 6:11 pm
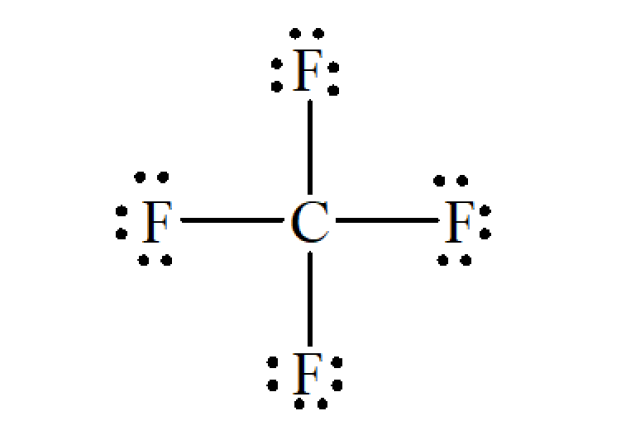
Refer to the structural formula of CF₄ below :
There are 4 C-F bond. Hence, number of bond pairs = 4
Each F atom has 3 lone pairs. Hence, number of lone pairs = 3 × 4 = 12
There are 4 C-F bond. Hence, number of bond pairs = 4
Each F atom has 3 lone pairs. Hence, number of lone pairs = 3 × 4 = 12
收錄日期: 2021-04-24 00:58:04
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20180311092623AA8zABi
