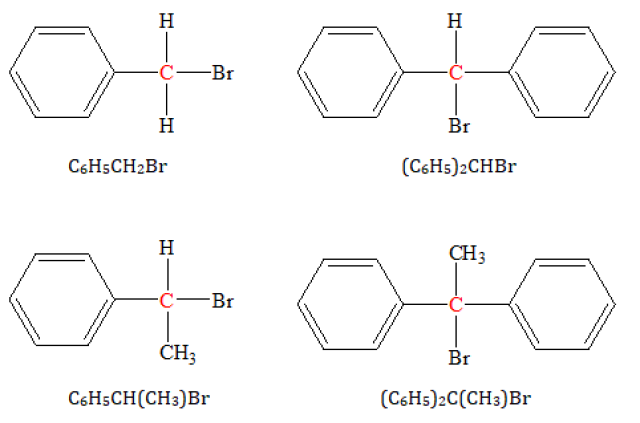
Classify into primary secondary or tertiary c6h5 ch2br, (c6h5)2 chbr, c6h5 ch(ch3)br, (c6h5)2 c(ch3)br?
2018-02-13 10:35 pm
回答 (3)
2018-02-14 1:38 am
✔ 最佳答案
The structures of the four compounds are shown below :C6H5CH2Br :
The carbon atom attached to the Br atom is also attached to 1 carbon atom.
Hence, it is primary bromide.
(C6H5)2CHBr :
The carbon atom attached to the Br atom is also attached to 2 carbon atoms.
Hence, it is secondary bromide.
C6H5CH(CH3)Br :
The carbon atom attached to the Br atom is also attached to 2 carbon atoms.
Hence, it is secondary bromide.
(C6H5)2C(CH3)Br :
The carbon atom attached to the Br atom is also attached to 3 carbon atoms.
Hence, it is tertiary bromide.
2018-02-14 12:30 am
in general
.. if the group is bonded to a C which is bonded to 1 other C (1 aklyl group), it's primary... 1°
.. if the group is bonded to a C which is bonded to 2 other C's (2 aklyl groups), it's secondary... 2°
.. if the group is bonded to a C which is bonded to 3 other C's (3 alkyl groups), it's tertiary... 3°
examples
.. ... ... ... .H
.. ... .. ... .. |
... . CH3 - C - OH... .. 1° alcohol... the OH is bonded to a C which is bonded to only 1 other C
.. ... .. ... .. |
.. ... ... ... .H
.. ... ... ... .CH3
.. ... .. ... .. |
... . CH3 - C - OH... .. 2° alcohol... the OH is bonded to a C which is bonded to 2 other C's
.. ... .. ... .. |
.. ... ... ... .H
.. ... ... ... .CH3
.. ... .. ... .. |
... . CH3 - C - OH... .. 3° alcohol... the OH is bonded to a C which is bonded to 3 other C's
.. ... .. ... .. |
.. ... ... ... .CH3
***********
***********
this problem.. .. it's complicated by the phenyl group.. C6H5. That's the whole point of this problem.
fyi...
.. (1) I'll use the symbol ϕ for the phenyl group.. C6H5-
.. (2) you need to note that one the phenyl group bonds like this
.. .. ..https://upload.wikimedia.org/wikipedia/commons/thumb/3/3b/Benzyl-cyanide-3D-balls.png/1200px-Benzyl-cyanide-3D-balls.png
.. .. ..the black spheres are carbon, white are hydrogen, blue is nitrogen
.. .. ..can you see the carbon - carbon bond between the phenyl ring and the central CH2 group?
you have these compounds..
.. ... ... .H
.. ... . .. |
... . ϕ - C - Br
.... ... .. |
.. ... ... .H
.. ... ... .ϕ
.. ... . .. |
... . ϕ - C - Br
.... ... .. |
.. ... ... .H
.. ... ... .CH3
.. ... . .. |
... . ϕ - C - Br
.... ... .. |
.. ... ... .H
.. ... ... .ϕ
.. ... . .. |
... . ϕ - C - Br
.... ... .. |
.. ... ... .CH3
*********
we can clearly see the CH3 is an alkyl group and adds a C-C bond. And we need to count that when determining 1°, 2, 3°. But we need to answer the question... "is the phenyl group considered a C-C bond (an alkyl group) that we need to consider when making the 2° and 3° determination".
********
the answer is related to whether or not the phenyl group imparts the same chemistry during reactions. SN1, SN2, E1, E2 as an alkyl group would. Get busy researching!
btw... the first compound is benzylbromide. Great place to start would be to google if benzylbromide, benzylalcohol, benzylchloride, etc are primary or secondary compounds. And how they react vs say.. methanol.. isopropyl alcohol.. etc
.. if the group is bonded to a C which is bonded to 1 other C (1 aklyl group), it's primary... 1°
.. if the group is bonded to a C which is bonded to 2 other C's (2 aklyl groups), it's secondary... 2°
.. if the group is bonded to a C which is bonded to 3 other C's (3 alkyl groups), it's tertiary... 3°
examples
.. ... ... ... .H
.. ... .. ... .. |
... . CH3 - C - OH... .. 1° alcohol... the OH is bonded to a C which is bonded to only 1 other C
.. ... .. ... .. |
.. ... ... ... .H
.. ... ... ... .CH3
.. ... .. ... .. |
... . CH3 - C - OH... .. 2° alcohol... the OH is bonded to a C which is bonded to 2 other C's
.. ... .. ... .. |
.. ... ... ... .H
.. ... ... ... .CH3
.. ... .. ... .. |
... . CH3 - C - OH... .. 3° alcohol... the OH is bonded to a C which is bonded to 3 other C's
.. ... .. ... .. |
.. ... ... ... .CH3
***********
***********
this problem.. .. it's complicated by the phenyl group.. C6H5. That's the whole point of this problem.
fyi...
.. (1) I'll use the symbol ϕ for the phenyl group.. C6H5-
.. (2) you need to note that one the phenyl group bonds like this
.. .. ..https://upload.wikimedia.org/wikipedia/commons/thumb/3/3b/Benzyl-cyanide-3D-balls.png/1200px-Benzyl-cyanide-3D-balls.png
.. .. ..the black spheres are carbon, white are hydrogen, blue is nitrogen
.. .. ..can you see the carbon - carbon bond between the phenyl ring and the central CH2 group?
you have these compounds..
.. ... ... .H
.. ... . .. |
... . ϕ - C - Br
.... ... .. |
.. ... ... .H
.. ... ... .ϕ
.. ... . .. |
... . ϕ - C - Br
.... ... .. |
.. ... ... .H
.. ... ... .CH3
.. ... . .. |
... . ϕ - C - Br
.... ... .. |
.. ... ... .H
.. ... ... .ϕ
.. ... . .. |
... . ϕ - C - Br
.... ... .. |
.. ... ... .CH3
*********
we can clearly see the CH3 is an alkyl group and adds a C-C bond. And we need to count that when determining 1°, 2, 3°. But we need to answer the question... "is the phenyl group considered a C-C bond (an alkyl group) that we need to consider when making the 2° and 3° determination".
********
the answer is related to whether or not the phenyl group imparts the same chemistry during reactions. SN1, SN2, E1, E2 as an alkyl group would. Get busy researching!
btw... the first compound is benzylbromide. Great place to start would be to google if benzylbromide, benzylalcohol, benzylchloride, etc are primary or secondary compounds. And how they react vs say.. methanol.. isopropyl alcohol.. etc
2018-02-13 10:44 pm
These are really best classified as variations of Benzylic bromides. The second and third choices would both be 2o, but they are WAY different with 1 vs 2 phenyl rings in play.
收錄日期: 2021-04-24 00:55:49
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20180213143526AAIbeUA
