What is the valencies of C in CO and CO2 And why?
2018-01-25 5:41 pm
回答 (1)
2018-01-25 6:11 pm
✔ 最佳答案
In chemistry, valency (or valence) is a measure of connectivity in bonding. The valency of an atom in a molecular compound can be determined by counting the number of chemical bonds excluding dative covalent bonds connected to this atom.The below diagram shows the bondings in CO and CO₂.
The valancy of C in CO = 2
The valency of C in CO₂ = 4
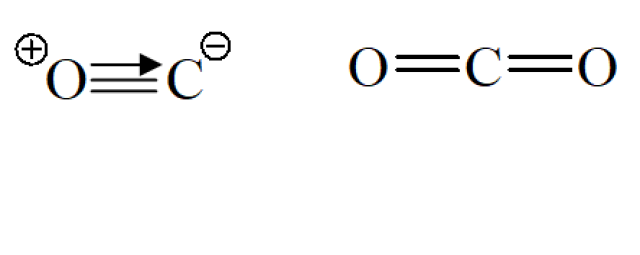
2018-01-26 12:24 am
Don't confuse "valence", "valency" and oxidation state. The valency is the maximum number of bonds that an element can have. So in all cases, the valency of carbon is 4. Chemists used to use the term "valence" where "oxidation state" is preferred now.
In the "olden days" we might say, "The valence of carbon in CO is +2, and in CO2 it is +4."
Today we would say, "The oxidation states of carbon in CO and CO2 are +2 and +4.
In the "olden days" we might say, "The valence of carbon in CO is +2, and in CO2 it is +4."
Today we would say, "The oxidation states of carbon in CO and CO2 are +2 and +4.
收錄日期: 2021-04-18 18:04:07
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20180125094158AAWJJTB
