Hi,
The equation for the reaction of ethene and bromine is:
C2H4(g) + Br2(l) C2H4Br2(l)
Bond energies
C—H 413
C ═ C 614
Br—Br 193
C—C 348
C—Br 276
So I did (614 + 193 + (4 × 413)) = 2459(kJ)
But the answer for products is:
348 + (2 × 276) + (4 × 413) = 2552(kJ)
I don t get it as a Br-Br bond is formed again? It broke in the first part?
Please can someone help?
Thanks
Stuck on a bond energies question. Can someone please help?
2018-01-24 10:36 am
回答 (2)
2018-01-24 10:58 am
Refer to the equation below.
Energy absorbed for bond breaking
= E(C=C) + 4E(C–H) + E(Br–Br)
= (614 + 4×413 + 193) kJ
= 2459 kJ
Energy released for bond formation
= E(C–C) + 2E(C–Br) + 4E(C–H)
= (348 + 2×276 + 4×413) kJ
= 2552 kJ
Heat of reaction, ΔH
= (2459 - 2552) kJ
= -93 kJ
Energy absorbed for bond breaking
= E(C=C) + 4E(C–H) + E(Br–Br)
= (614 + 4×413 + 193) kJ
= 2459 kJ
Energy released for bond formation
= E(C–C) + 2E(C–Br) + 4E(C–H)
= (348 + 2×276 + 4×413) kJ
= 2552 kJ
Heat of reaction, ΔH
= (2459 - 2552) kJ
= -93 kJ
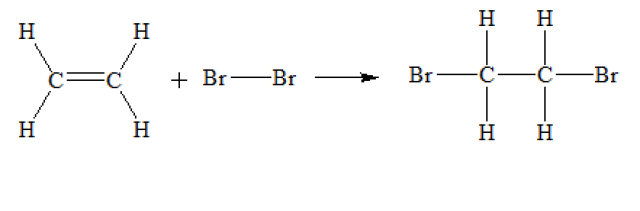
2018-01-24 10:56 am
Here's the reaction: H2C=CH2 + Br2 --> BrH2C-CH2Br There is no Br2 in the product.
There is no elemental chlorine in CaCl2 either.
There is no elemental chlorine in CaCl2 either.
收錄日期: 2021-04-18 18:02:44
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20180124023651AAcFGff
