AP Chemistry Question?
2018-01-22 12:54 pm
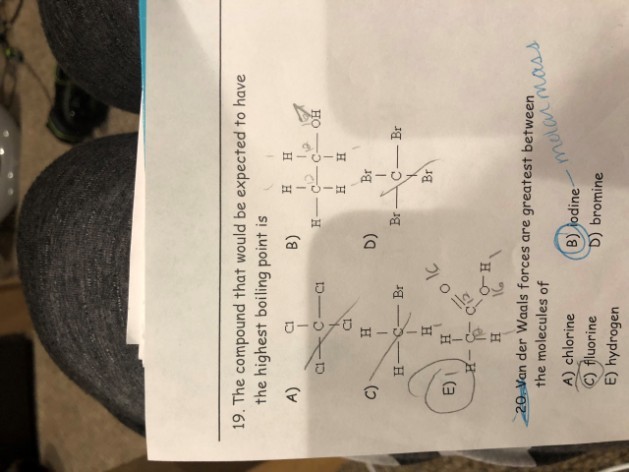
Question 19
回答 (2)
2018-01-22 4:05 pm
✔ 最佳答案
The molecules of CH₃CH₂OH (option B) are held together by hydrogen bonds, while the molecules of the other three options are held together by van der Waals' forces.For small molecules, hydrogen bonds are stronger than van der Waals' forces. Therefore, among the four options, CH₃CH₂OH has the strongest intermolecular forces and thus has the highest boiling point.
The answers: B) CH₃–CH₂–OH
2018-01-22 3:35 pm
D) CBr4
收錄日期: 2021-04-18 18:03:02
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20180122045431AARdlQ1

