Does copper or zinc have a higher melting point?
2018-01-19 12:36 pm
回答 (3)
2018-01-19 3:07 pm
Copper has a higher melting point than zinc.
The electron configurations :
Cu : 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁹ 4s²
Zn : 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s²
In melting of each of the two metals, energy is absorbed to overcome a part of the metallic bonds of the metal. As the 3d subshell of zinc is completely filled, it exhibits extra stability and thus the valence 3d electrons are less available for delocalization to make the metallic bonds. Therefore, the metallic bonds of zinc are weaker than the metallic bonds of copper.
As the metallic bonds of copper are stronger than the metallic bonds of zinc, more energy is needed to overcome the metallic bonds of copper. Therefore, copper has a higher melting point.
If you have not learned s, p, d, f orbitals of atoms, neglect the above explanation.
The electron configurations :
Cu : 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁹ 4s²
Zn : 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s²
In melting of each of the two metals, energy is absorbed to overcome a part of the metallic bonds of the metal. As the 3d subshell of zinc is completely filled, it exhibits extra stability and thus the valence 3d electrons are less available for delocalization to make the metallic bonds. Therefore, the metallic bonds of zinc are weaker than the metallic bonds of copper.
As the metallic bonds of copper are stronger than the metallic bonds of zinc, more energy is needed to overcome the metallic bonds of copper. Therefore, copper has a higher melting point.
If you have not learned s, p, d, f orbitals of atoms, neglect the above explanation.
2018-01-19 12:42 pm
Copper by far, at about 1984 F° while zinc is only at around 787 F°.
2018-01-19 1:30 pm
Higher melting point....
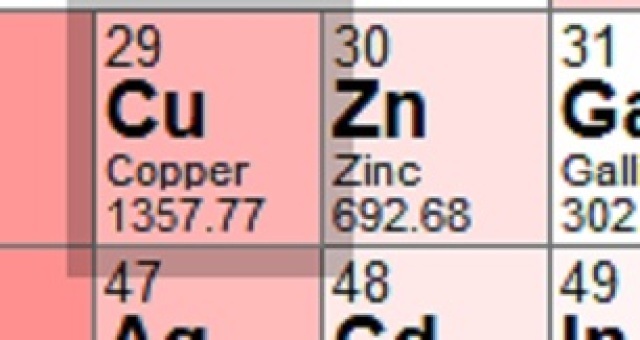
Did it occur to you that you could easily find this information using any number of resources? Looking up copper and zinc on Wikipedia is one way. Or you could look at www.ptable.com, as I did. It's a shame that some people want everything done for them, rather than doing a bit of work on their own.
So in case you are incapable of using the computer on your own, here are the melting points from www.ptable.com. And by the way, since this is a chemistry forum, the temperatures are in Kelvin degrees. I'll leave it to you to convert to some other scale.
========= Follow up =========
As for the "why" of the greater melting point of copper, I'm not sure I would explain it based solely on the electron configuration as 不用客氣 has suggested. In fact, we need to get the electron configuration correct.
Cu ..... [Ar] 3d10, 4s1 ............. copper is an exception to the Aufbau principle
Zn ..... [Ar] 3d10, 4s2
Is that really enough difference to account for the difference in bond energies and melting points? Consider the fact that copper (a slightly lighter atom) has a greater density than zinc (8.92 g/cm³ for Cu, vs 7.14 g/cm³ for Zn). This means that the copper atoms are closer together in the metallic crystal lattice and according to Coulomb's law, the shorter distance will mean a stronger force of attraction between each nucleus and all of the neighboring electrons (F = kq1q2 / r²) and a higher melting point for copper.
The pattern is periodic. The elements in group 11 (Cu, Ag, Au) have both greater melting points and greater densities than the slightly heavier elements in group 12 (Zn, Cd, Hg). Of course, the electron configurations are also periodic with Cu, Ag and Au each have an (n-1)d10, ns1 arrangement, compared to (n-1)d10, ns2 for the elements in group 12.
.
Did it occur to you that you could easily find this information using any number of resources? Looking up copper and zinc on Wikipedia is one way. Or you could look at www.ptable.com, as I did. It's a shame that some people want everything done for them, rather than doing a bit of work on their own.
So in case you are incapable of using the computer on your own, here are the melting points from www.ptable.com. And by the way, since this is a chemistry forum, the temperatures are in Kelvin degrees. I'll leave it to you to convert to some other scale.
========= Follow up =========
As for the "why" of the greater melting point of copper, I'm not sure I would explain it based solely on the electron configuration as 不用客氣 has suggested. In fact, we need to get the electron configuration correct.
Cu ..... [Ar] 3d10, 4s1 ............. copper is an exception to the Aufbau principle
Zn ..... [Ar] 3d10, 4s2
Is that really enough difference to account for the difference in bond energies and melting points? Consider the fact that copper (a slightly lighter atom) has a greater density than zinc (8.92 g/cm³ for Cu, vs 7.14 g/cm³ for Zn). This means that the copper atoms are closer together in the metallic crystal lattice and according to Coulomb's law, the shorter distance will mean a stronger force of attraction between each nucleus and all of the neighboring electrons (F = kq1q2 / r²) and a higher melting point for copper.
The pattern is periodic. The elements in group 11 (Cu, Ag, Au) have both greater melting points and greater densities than the slightly heavier elements in group 12 (Zn, Cd, Hg). Of course, the electron configurations are also periodic with Cu, Ag and Au each have an (n-1)d10, ns1 arrangement, compared to (n-1)d10, ns2 for the elements in group 12.
.
收錄日期: 2021-04-18 18:06:08
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20180119043604AAWt6Jr
