Chemistry Questions...?
2018-01-16 8:52 pm
An Organic compound A having molecular formula C2H3N on complete reduction with nascent hydrogen gave another compound B and the compound B on treatment with Nitrous Acid gave ethanol. Write the equations involved to identify A and B.
回答 (2)
2018-01-16 10:17 pm
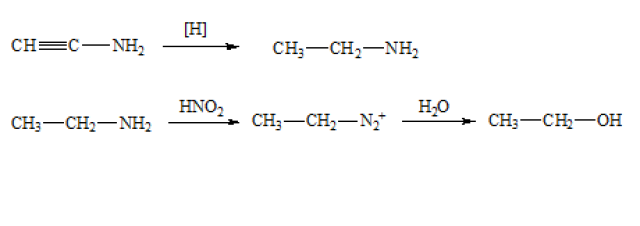
A is an unsaturated organic nitrogenous compound. When compound A is completely reduced by nascent hydrogen, it would give a saturated organic nitrogenous compound C₂H₇N. Therefore, the molecular formula of compound B is C₂H₇N.
When compound B is treated with nitrous acid, it gave ethanol. It can be deduced that compound B is an amine, i.e. CH₃CH₂NH₂.
Then, it can be deduced that the compound A is an unsaturated amine with molecular formula C₂H₃N. Hence, compound A is CH≡C–NH₂.
The equations are shown below:
When compound B is treated with nitrous acid, it gave ethanol. It can be deduced that compound B is an amine, i.e. CH₃CH₂NH₂.
Then, it can be deduced that the compound A is an unsaturated amine with molecular formula C₂H₃N. Hence, compound A is CH≡C–NH₂.
The equations are shown below:
2018-01-17 12:18 am
Much more reasonably, the starting compound is acetonitrile: CH3C///N
收錄日期: 2021-04-24 00:55:22
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20180116125209AAKzKb1
