Chemistry?
2018-01-12 9:05 pm
Elementary Reactions

回答 (2)
2018-01-13 1:37 am
✔ 最佳答案
44.The steric factor in the second reaction is more important.
In the second reaction, NO is a diatomic molecule with two different atoms. Reaction occurs when both NO molecule and O₃ molecule are collided in specific orientations.
In the first reaction, Cl is a spherical atom. Reaction occurs when O₃ molecule is collided in a specific orientation, and it is not necessary to consider the orientation of Cl atom.
====
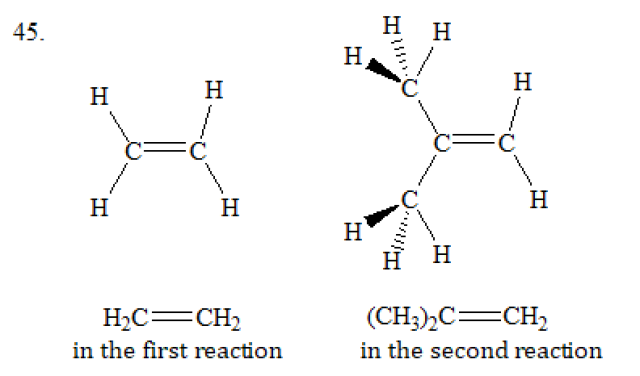
45.
The steric factor in the second reaction is more important.
The diagram below shows the structures of H₂C=CH₂ and (CH₃)₂C=CH₂. (CH₃)₂C=CH₂ is more steric hindrance than H₂C=CH₂. This is because the two -CH₃ groups in (CH₃)₂C=CH₂ are much more bulky that the two H atoms in H₂C=CH₂. Therefore, the steric factor in the second reaction is more important.
2018-01-13 12:34 am
# 45
you have this reaction
.. H.. ... .. H
.. ..\.. .. ../
.. .. C = C + H - H ---- -----> CH3 - CH3
.. .. /.. .. .\
.. H.. .. ...H
vs
.. .. ..H
.. .. ..|
.H -.C - H... H
.. .....\.. ... ../
.. .. .. C = C + H - Br ---- -----> (CH3)2CH-CH2Br & (CH3)2CBr-CH3
.. .. .. /.. .. .\
.H - C - H...H
.... . |
.. .. .H
you tell me.. which of those has more "steric hinderence" effects>
you have this reaction
.. H.. ... .. H
.. ..\.. .. ../
.. .. C = C + H - H ---- -----> CH3 - CH3
.. .. /.. .. .\
.. H.. .. ...H
vs
.. .. ..H
.. .. ..|
.H -.C - H... H
.. .....\.. ... ../
.. .. .. C = C + H - Br ---- -----> (CH3)2CH-CH2Br & (CH3)2CBr-CH3
.. .. .. /.. .. .\
.H - C - H...H
.... . |
.. .. .H
you tell me.. which of those has more "steric hinderence" effects>
收錄日期: 2021-04-24 00:55:17
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20180112130533AADljN2
