How can i determine planar molcules?
2017-12-24 1:28 am
回答 (2)
2017-12-24 2:31 am
Planar molecules
Those which are linear or bent must lie in a single plane. So if there are three atoms total, the molecule is either linear or bent and therefore planar.
Here is a summary of VSEPR theory
#electron pairs ..... e- pair geom..... molecular geom..... bond angle
2 ........................... linear ..................... linear .................... 180
3 (no lone pairs) .. trigonal planar ...... trigonal planar ....... 120
3 (one lone pair) .. trigonal planar ...... bent .........................LT 120
4 (no lone pairs) .. tetrahedral ............ tetrahedral .............. 109.5
4 (one lone pair) .. tetrahedral ............ trigonal pyramidal .. LT 109.5
4 (2 lone pairs) .... tetrahedral ............ bent ...................….. LT. 109.5
5 (no lone pairs) .. trig bipyramidal ....trig bipyramidal ….. 90 and 120
5 (one lone pair) .. trig bipyramidal ... see-saw ................... 90 and 120
5 (2 lone pairs) .... trig bipyramidal ....T-shaped .............,... 90
5 (3 lone pairs) .... trig bipyramidal ....linear ........................180
6 (no lone pairs) .. octahedral ............ octahedral ................ 90
6 (one lone pair) .. octahedral ............ square pyramidal ......90
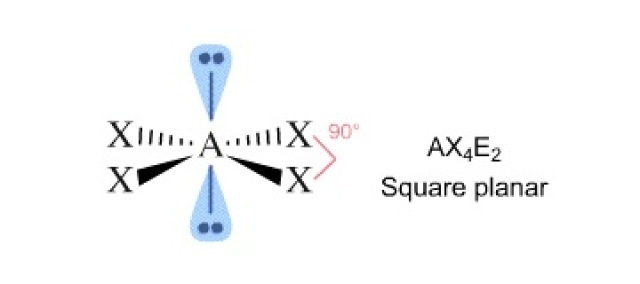
6 (2 lone pairs) .... octahedral ............ square planar .............90
(LT = "less than")
You are probably more interested in "trigonal planar" or "square planar." A trigonal planar molecule has four atoms, and the central atom does not have any lone pairs. Example: BCl3.
A square planar molecule has five atoms and two lone pairs on the central atom. Example: XeF4
Those which are linear or bent must lie in a single plane. So if there are three atoms total, the molecule is either linear or bent and therefore planar.
Here is a summary of VSEPR theory
#electron pairs ..... e- pair geom..... molecular geom..... bond angle
2 ........................... linear ..................... linear .................... 180
3 (no lone pairs) .. trigonal planar ...... trigonal planar ....... 120
3 (one lone pair) .. trigonal planar ...... bent .........................LT 120
4 (no lone pairs) .. tetrahedral ............ tetrahedral .............. 109.5
4 (one lone pair) .. tetrahedral ............ trigonal pyramidal .. LT 109.5
4 (2 lone pairs) .... tetrahedral ............ bent ...................….. LT. 109.5
5 (no lone pairs) .. trig bipyramidal ....trig bipyramidal ….. 90 and 120
5 (one lone pair) .. trig bipyramidal ... see-saw ................... 90 and 120
5 (2 lone pairs) .... trig bipyramidal ....T-shaped .............,... 90
5 (3 lone pairs) .... trig bipyramidal ....linear ........................180
6 (no lone pairs) .. octahedral ............ octahedral ................ 90
6 (one lone pair) .. octahedral ............ square pyramidal ......90
6 (2 lone pairs) .... octahedral ............ square planar .............90
(LT = "less than")
You are probably more interested in "trigonal planar" or "square planar." A trigonal planar molecule has four atoms, and the central atom does not have any lone pairs. Example: BCl3.
A square planar molecule has five atoms and two lone pairs on the central atom. Example: XeF4
2017-12-24 2:21 am
Draw their Lewis structures and look at the number of pairs of electrons to determine the geometry (VSEPR)
收錄日期: 2021-05-03 01:41:39
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20171223172844AAEjWle
