S8 is not a flat octagon - Why?
2017-11-01 11:20 am
回答 (2)
2017-11-01 11:54 am
✔ 最佳答案
There are many allotropes of sulfur. One of the most common forms of sulfur is the S8 "crown". It forms the crown structure because of the arrangement of the bonding and nonbonding pairs (lone pairs) of electrons. The pairs of electrons will arrange themselves so they are as far apart as possible to minimize repulsion (VSEPR theory).(Thanks Wikipedia for the picture below.)
.
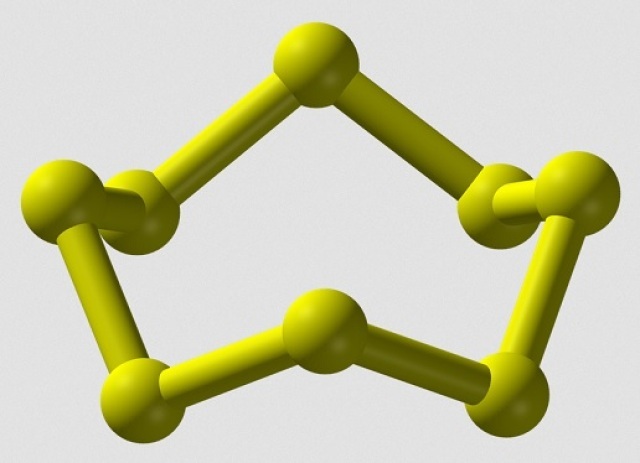
2017-11-01 11:51 am
In S₈ molecule, each S atom is attached to 2 bond pairs of electrons and 2 lone pairs. The 4 electron pairs are arranged tetrahedrally, and thus the bond angle is about 107°.
For a flat regular octagon, each interior angle = (8 - 2) × 180° / 8 = 135°
Obviously, the difference between the bond angle and each interior angle of flat octagon is quite great. Therefore, S₈ is not flat octagon.
The molecular shape of S₈ is shown below.
For a flat regular octagon, each interior angle = (8 - 2) × 180° / 8 = 135°
Obviously, the difference between the bond angle and each interior angle of flat octagon is quite great. Therefore, S₈ is not flat octagon.
The molecular shape of S₈ is shown below.
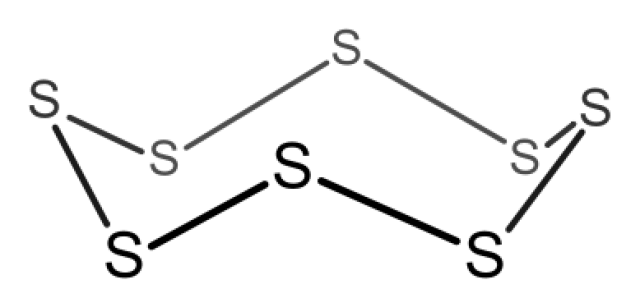
收錄日期: 2021-04-18 17:55:34
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20171101032029AAh2tG3
