I asked my teacher to explain why that's the answer but she just told me its because the final state here is defined to be the first excited state.
But I'm still confuse
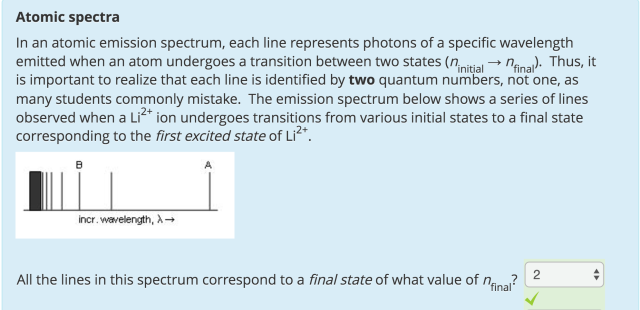
Atomic Spectra: Why is the answer 2? 10 ptss?
2017-10-22 4:52 pm
回答 (1)
2017-10-22 5:42 pm
Each Li atom has 3 electrons, and a Li atom loses 2 electrons to become a Li²⁺ ion.
Hence, Li²⁺ has only 1 electron and thus only 1 electron shell is occupies.
The ground state : the electron is in the shell with n = 1
The first excited state : the electron is in the shell with n = 2
The second excited state : the electron is in the shell with n = 3
…… (and so on) ……
Consider the spectrum in the question.
The lines corresponding to "various initial states" → "a final state"
The final state is corresponding to the "first excited state of Li²⁺".
Hence, the final state corresponding to "the shell with n = 2".
Hence, Li²⁺ has only 1 electron and thus only 1 electron shell is occupies.
The ground state : the electron is in the shell with n = 1
The first excited state : the electron is in the shell with n = 2
The second excited state : the electron is in the shell with n = 3
…… (and so on) ……
Consider the spectrum in the question.
The lines corresponding to "various initial states" → "a final state"
The final state is corresponding to the "first excited state of Li²⁺".
Hence, the final state corresponding to "the shell with n = 2".
收錄日期: 2021-04-24 00:51:24
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20171022085254AApFUnX
