Question 4 options:
7
9
5
6
8
In the Lewis structure of SOF2, the total number of electron lone pairs is?
2017-10-18 11:32 am
回答 (2)
2017-10-18 11:54 am
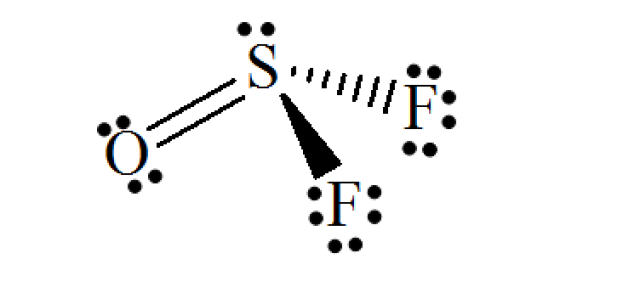
Refer to the structure of SOF₂ below.
Number of electron lone pairs on the S atom = 1
Number of electron lone pairs on the O atom = 2
Number of electron lone pairs on the two F atoms = 3 × 2 = 6
Total number of electron lone pairs = 1 + 2 + 6 = 9
The answer: 9
Number of electron lone pairs on the S atom = 1
Number of electron lone pairs on the O atom = 2
Number of electron lone pairs on the two F atoms = 3 × 2 = 6
Total number of electron lone pairs = 1 + 2 + 6 = 9
The answer: 9
2017-10-18 11:48 am
There are 9 lone pairs in SOF2.
SOF2 does not follow the octet rule. Sulfur, the central atom, has an "expanded octet" with 10 electrons around it.
......••
F − S = O .............. Oxygen has two lone pairs
.......|
...... F...................... Each fluorine has three lone pairs
If you give each element an octet by having a single bond between S and O, then the formal charge on S is +1 and O is -1. With the double bond, all the formal charges are zero as is preferred for non-period 2 central atom.
SOF2 does not follow the octet rule. Sulfur, the central atom, has an "expanded octet" with 10 electrons around it.
......••
F − S = O .............. Oxygen has two lone pairs
.......|
...... F...................... Each fluorine has three lone pairs
If you give each element an octet by having a single bond between S and O, then the formal charge on S is +1 and O is -1. With the double bond, all the formal charges are zero as is preferred for non-period 2 central atom.
收錄日期: 2021-04-18 17:56:31
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20171018033201AAz1grX
