Fructose is ketonic while glucose aldehydic....Even Schiff s reagent reacts with aldehydes only...
But my class notes say it cannot distinguish them???
Why Schiff s reagent can t distinguish between glucose and fructose???
2017-08-07 8:28 pm
回答 (1)
2017-08-08 1:40 am
✔ 最佳答案
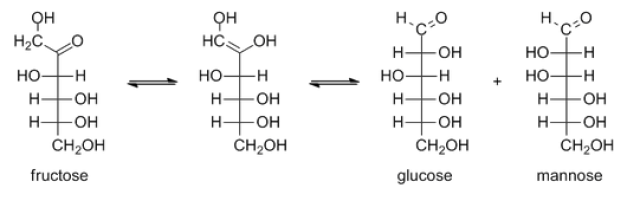
Schiff's reagent can be used for detection of many organic aldehydes that has also found use in the staining of biological tissues. Such method is known as Schiff's test.Although fructose is a ketose, it can give a positive result in the Schiff's test. This is because fructose is readily isomerized (such isomerization is known as tautomerism) to a mixture of glucose and mannose under basic conditions. Both glucose and mannose are aldoses and thus give positive result in Schiff's test. The equation of such isomerization is shown below.
收錄日期: 2021-04-18 17:34:30
原文連結 [永久失效]:
https://hk.answers.yahoo.com/question/index?qid=20170807122817AAyQhnL
